当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular preparation of biphenyl triazoles via click chemistry as non-competitive hyaluronidase inhibitors
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.bioorg.2024.107291 Yiman Qin , Guanyi Li , Ling Wang , Guangyuan Yin , Xiang Zhang , Hongxiang Wang , Pengfei Zheng , Wentao Hua , Yan Cheng , Yaxue Zhao , Jiong Zhang
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.bioorg.2024.107291 Yiman Qin , Guanyi Li , Ling Wang , Guangyuan Yin , Xiang Zhang , Hongxiang Wang , Pengfei Zheng , Wentao Hua , Yan Cheng , Yaxue Zhao , Jiong Zhang
|
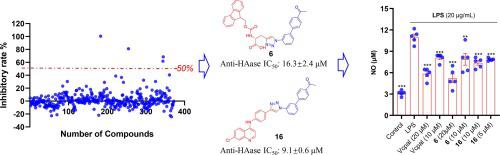
Hyaluronidase is a promising target in drug discovery, given its overexpression in a range of physiological and pathological processes, including tumor migration, skin aging, sagging, and wrinkling, as well as inflammation and bacterial infections. In this study, to identify novel hyaluronidase inhibitors, we applied click chemistry for the modular synthesis of 370 triazoles in 96-well plates, starting with biphenyl azide. Utilizing an optimized turbidimetric screening assay in microplates, we identified Fmoc-containing triazoles and , as well as quinoline-containing triazoles and , as highly effective hyaluronidase inhibitors. Subsequent research indicated that these triazoles potentially interact with a novel binding site of hyaluronidase. Notably, these inhibitors displayed minimal cytotoxicity and showed promising anti-inflammatory effects in LPS-stimulated macrophages. Remarkably, compound significantly reduced NO release by 74 % at a concentration of 20 μM.
中文翻译:

通过点击化学模块化制备联苯三唑作为非竞争性透明质酸酶抑制剂
透明质酸酶是药物发现中一个有前途的靶点,因为它在一系列生理和病理过程中过度表达,包括肿瘤迁移、皮肤老化、下垂和皱纹,以及炎症和细菌感染。在本研究中,为了鉴定新型透明质酸酶抑制剂,我们应用点击化学在 96 孔板中模块化合成 370 个三唑,从联苯叠氮化物开始。利用微孔板中优化的比浊筛选试验,我们鉴定出含 Fmoc 的三唑类和 ,以及含喹啉的三唑类和 ,作为高效的透明质酸酶抑制剂。随后的研究表明这些三唑可能与透明质酸酶的新结合位点相互作用。值得注意的是,这些抑制剂表现出最小的细胞毒性,并在 LPS 刺激的巨噬细胞中显示出有希望的抗炎作用。值得注意的是,浓度为 20 μM 的化合物可显着减少 74% 的 NO 释放。
更新日期:2024-03-16
中文翻译:

通过点击化学模块化制备联苯三唑作为非竞争性透明质酸酶抑制剂
透明质酸酶是药物发现中一个有前途的靶点,因为它在一系列生理和病理过程中过度表达,包括肿瘤迁移、皮肤老化、下垂和皱纹,以及炎症和细菌感染。在本研究中,为了鉴定新型透明质酸酶抑制剂,我们应用点击化学在 96 孔板中模块化合成 370 个三唑,从联苯叠氮化物开始。利用微孔板中优化的比浊筛选试验,我们鉴定出含 Fmoc 的三唑类和 ,以及含喹啉的三唑类和 ,作为高效的透明质酸酶抑制剂。随后的研究表明这些三唑可能与透明质酸酶的新结合位点相互作用。值得注意的是,这些抑制剂表现出最小的细胞毒性,并在 LPS 刺激的巨噬细胞中显示出有希望的抗炎作用。值得注意的是,浓度为 20 μM 的化合物可显着减少 74% 的 NO 释放。



























 京公网安备 11010802027423号
京公网安备 11010802027423号