当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Density, Vapor Pressure, Enthalpy of Vaporization, and Heat Capacity of (E,E)-Methyl Sorbate
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.jced.3c00695 Jumei Xu 1 , Yu Qi 1 , Zuoxiang Zeng 1 , Weilan Xue 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.jced.3c00695 Jumei Xu 1 , Yu Qi 1 , Zuoxiang Zeng 1 , Weilan Xue 1
Affiliation
|
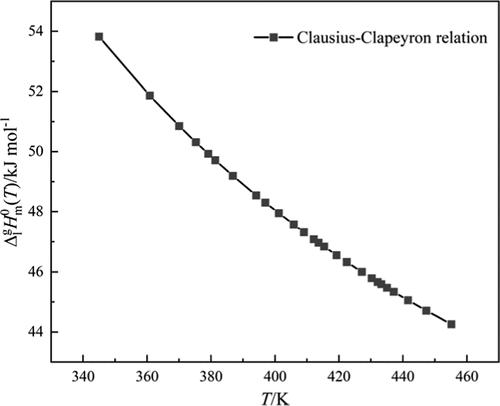
The density and the heat capacity of (E,E)-methyl sorbate (MS) were measured from 286.35 to 433.25 K using a pycnometer and an adiabatic calorimeter, respectively. The saturated vapor pressure of MS was determined by the ebulliometric method from 344.95 to 455.15 K and correlated by the Antoine equation with an average relative deviation of 1.68%. The enthalpy of vaporization of MS at the normal boiling point was figured out to be 44.29 kJ mol–1 according to the Clausius–Clapeyron equation. Based on the Othmer method, the standard enthalpy of vaporization of MS was obtained using three substances as a reference, and the calculated results were further verified by the Watson relation and the Clarke–Glew equation.
中文翻译:

(E,E)-山梨酸甲酯的密度、蒸气压、汽化焓和热容
使用比重瓶和绝热量热计分别在 286.35 至 433.25 K 范围内测量( E , E )-山梨酸甲酯 (MS)的密度和热容。通过沸点法测定了MS的饱和蒸气压,范围为344.95至455.15 K,并通过Antoine方程进行关联,平均相对偏差为1.68%。根据Clausius-Clapeyron方程计算出MS在常沸点的汽化焓为44.29 kJ mol –1 。基于Othmer方法,以三种物质为参考,得到了MS的标准汽化焓,并通过Watson关系式和Clarke-Glew方程进一步验证了计算结果。
更新日期:2024-03-20
中文翻译:

(E,E)-山梨酸甲酯的密度、蒸气压、汽化焓和热容
使用比重瓶和绝热量热计分别在 286.35 至 433.25 K 范围内测量( E , E )-山梨酸甲酯 (MS)的密度和热容。通过沸点法测定了MS的饱和蒸气压,范围为344.95至455.15 K,并通过Antoine方程进行关联,平均相对偏差为1.68%。根据Clausius-Clapeyron方程计算出MS在常沸点的汽化焓为44.29 kJ mol –1 。基于Othmer方法,以三种物质为参考,得到了MS的标准汽化焓,并通过Watson关系式和Clarke-Glew方程进一步验证了计算结果。



























 京公网安备 11010802027423号
京公网安备 11010802027423号