当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental Solubility and Absorption Mechanism of Dilute SO2 in the Binary System of Diethylene Glycol Monomethyl Ether and Dimethyl Sulfoxide
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.jced.3c00657 Jia Liu 1, 2, 3 , Qiaomin Zhang 1, 2, 3 , Huifang Guo 1, 2, 3 , Jianbin Zhang 4 , Xiaohong Xie 1, 2, 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.jced.3c00657 Jia Liu 1, 2, 3 , Qiaomin Zhang 1, 2, 3 , Huifang Guo 1, 2, 3 , Jianbin Zhang 4 , Xiaohong Xie 1, 2, 3
Affiliation
|
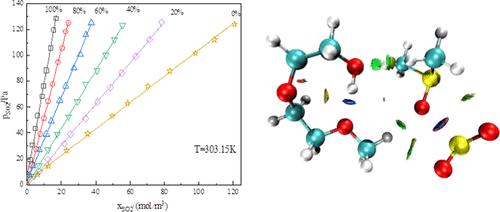
In this paper, a diethylene glycol monomethyl ether (DGME) + dimethyl sulfoxide (DMSO) system was used for the absorption of dilute SO2, and the gas–liquid equilibrium (GLE) data of the binary system for SO2 at p = 123.15 kPa and T= (303.15 K-318.15 K) were measured by a GLE device. Meanwhile, different temperatures and different ratios were also measured, Henry’s constants at different temperatures were fitted based on the measured data, and the thermodynamic parameters of dissolution enthalpy, dissolution entropy, and dissolution Gibbs free energy of the dissolution process were calculated. The results of five cycles of SO2 absorption and resolution showed that the maximum absorption of SO2 in the binary system was 0.85 g/g. The desorption rate could reach 97%, and the absorption and desorption capacities did not decrease significantly after the cycle. The mechanism of SO2 absorption in the binary system was discussed and analyzed by combining spectral analysis and quantum chemistry. The spectroscopic results show that the weak interaction forces between DGME, DMSO, and SO2 are hydrogen bonding forces and dipole–dipole interaction forces, which lay the theoretical foundation for the application and practice of binary hybrid systems. This can provide important GLE data for the design and operation of organic solvent absorption and desorption of SO2 in flue gas desulfurization, and the prepared absorbent has a potential industrial application value.
中文翻译:

稀SO2在二乙二醇单甲醚和二甲亚砜二元体系中的溶解度及吸收机理实验
本文采用二甘醇单甲醚(DGME)+二甲基亚砜(DMSO)体系吸收稀SO 2 ,得到SO 2二元体系在p = 123.15时的气液平衡(GLE)数据kPa 和T = (303.15 K-318.15 K) 通过 GLE 设备测量。同时还测量了不同温度和不同比例,根据测量数据拟合不同温度下的亨利常数,计算出溶解过程的溶解热力学参数溶解焓、溶解熵和溶解吉布斯自由能。 5个循环的SO 2吸收解析结果表明,二元体系中SO 2的最大吸收量为0.85 g/g。解吸率可达97%,且循环后吸附和解吸容量没有明显下降。结合光谱分析和量子化学,讨论和分析了二元体系中SO 2吸收的机理。光谱结果表明DGME、DMSO和SO 2之间的弱相互作用力是氢键力和偶极-偶极相互作用力,这为二元杂化体系的应用和实践奠定了理论基础。这可为烟气脱硫中有机溶剂吸收和解吸SO 2的设计和运行提供重要的GLE数据,制备的吸收剂具有潜在的工业应用价值。
更新日期:2024-03-20
中文翻译:

稀SO2在二乙二醇单甲醚和二甲亚砜二元体系中的溶解度及吸收机理实验
本文采用二甘醇单甲醚(DGME)+二甲基亚砜(DMSO)体系吸收稀SO 2 ,得到SO 2二元体系在p = 123.15时的气液平衡(GLE)数据kPa 和T = (303.15 K-318.15 K) 通过 GLE 设备测量。同时还测量了不同温度和不同比例,根据测量数据拟合不同温度下的亨利常数,计算出溶解过程的溶解热力学参数溶解焓、溶解熵和溶解吉布斯自由能。 5个循环的SO 2吸收解析结果表明,二元体系中SO 2的最大吸收量为0.85 g/g。解吸率可达97%,且循环后吸附和解吸容量没有明显下降。结合光谱分析和量子化学,讨论和分析了二元体系中SO 2吸收的机理。光谱结果表明DGME、DMSO和SO 2之间的弱相互作用力是氢键力和偶极-偶极相互作用力,这为二元杂化体系的应用和实践奠定了理论基础。这可为烟气脱硫中有机溶剂吸收和解吸SO 2的设计和运行提供重要的GLE数据,制备的吸收剂具有潜在的工业应用价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号