当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
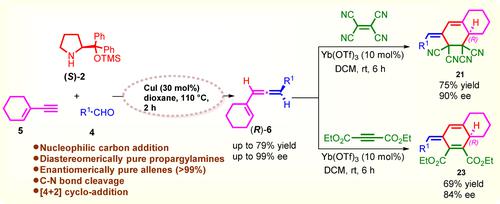
Copper(I)-Catalyzed Enantioselective Synthesis of Chiral Cyclohexenylallenes and Transfer of Axial to Center Chirality by Ytterbium (III) Catalyzed [4+2] Cycloaddition
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-21 , DOI: 10.1002/ajoc.202300665 Iddum Satyanarayana 1 , Mohan Lakavathu 2 , Mariappan Periasamy 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-21 , DOI: 10.1002/ajoc.202300665 Iddum Satyanarayana 1 , Mohan Lakavathu 2 , Mariappan Periasamy 1
Affiliation
|
Chiral cyclohexenylallenes are readily accessed from 1-enynes, aldehydes and chiral secondary amines in good yields (up to 79 % yield) with excellent enantioselectivities (up to 99 % ee) in dioxane at 110° C, through CuI catalyzed the formation of chiral propargylamine intermediates. Subsequent Yb(OTf)3 catalyzed [4+2] cycloaddition reaction of cyclohexylallenes with the dienophiles tetracyanoethylene and diethyl acetylenedicarboxylate afforded the corresponding bicyclic products in 69–75 % yield and 84–90 % ee.
中文翻译:

铜(I)催化对映选择性合成手性环己烯基丙二烯及镱(III)催化[4+2]环加成将轴向手性转移到中心手性
手性环己烯基联二烯可以很容易地从 1-烯炔、醛和手性仲胺得到,通过 CuI 催化形成手性炔丙胺,在二恶烷中于 110°C 下以良好的产率(高达 79% 产率)和优异的对映选择性(高达 99% ee)获得手性环己烯基联烯中间体。随后,Yb(OTf) 3催化环己基丙二烯与亲二烯体四氰乙烯和乙炔二甲酸二乙酯发生[4+2]环加成反应,得到相应的双环产物,收率 69–75%,ee 84–90%。
更新日期:2024-03-21
中文翻译:

铜(I)催化对映选择性合成手性环己烯基丙二烯及镱(III)催化[4+2]环加成将轴向手性转移到中心手性
手性环己烯基联二烯可以很容易地从 1-烯炔、醛和手性仲胺得到,通过 CuI 催化形成手性炔丙胺,在二恶烷中于 110°C 下以良好的产率(高达 79% 产率)和优异的对映选择性(高达 99% ee)获得手性环己烯基联烯中间体。随后,Yb(OTf) 3催化环己基丙二烯与亲二烯体四氰乙烯和乙炔二甲酸二乙酯发生[4+2]环加成反应,得到相应的双环产物,收率 69–75%,ee 84–90%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号