当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
WTAP-induced N6-methyladenosine of PD-L1 blocked T-cell-mediated antitumor activity under hypoxia in colorectal cancer
Cancer Science ( IF 5.7 ) Pub Date : 2024-03-20 , DOI: 10.1111/cas.16136 Qi‐zhi Liu 1 , Nan Zhang 1 , Jun‐yi Chen 1 , Min‐jun Zhou 1 , De‐hua Zhou 1 , Zhuo Chen 1 , Zhen‐xing Huang 1 , Yu‐xiang Xie 1 , Guang‐lei Qiao 2 , Xiao‐huang Tu 1
Cancer Science ( IF 5.7 ) Pub Date : 2024-03-20 , DOI: 10.1111/cas.16136 Qi‐zhi Liu 1 , Nan Zhang 1 , Jun‐yi Chen 1 , Min‐jun Zhou 1 , De‐hua Zhou 1 , Zhuo Chen 1 , Zhen‐xing Huang 1 , Yu‐xiang Xie 1 , Guang‐lei Qiao 2 , Xiao‐huang Tu 1
Affiliation
|
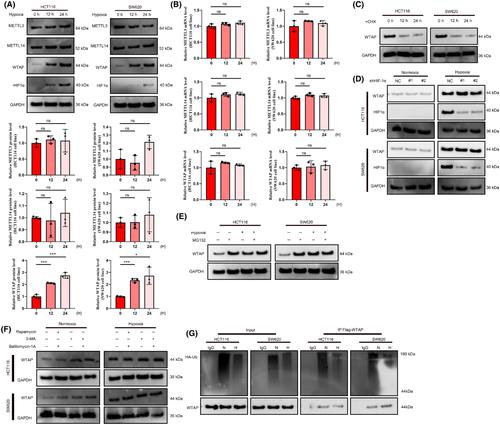
N6-Methyladenosine (m6A) is a important process regulating gene expression post-transcriptionally. Programmed death ligand 1 (PD-L1) is a major immune inhibitive checkpoint that facilitates immune evasion and is expressed in tumor cells. In this research we discovered that Wilms' tumor 1-associated protein (WTAP) degradation caused by ubiquitin-mediated cleavage in cancer cells (colorectal cancer, CRC) under hypoxia was inhibited by Pumilio homolog 1 (PUM1) directly bound to WTAP. WTAP enhanced PD-L1 expression in a way that was m6A-dependent. m6A “reader,” Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) identified methylated PD-L1 transcripts and subsequently fixed its mRNA. Additionally, we found that T-cell proliferation and its cancer cell-killing effects were prevented by overexpression of WTAP in vitro and in vivo. Overexpression prevented T cells from proliferating and killing CRC by maintaining the expression of PD-L1. Further evidence supporting the WTAP–PD-L1 regulatory axis was found in human CRC and organoid tissues. Tumors with high WTAP levels appeared more responsive to anti-PD1 immunotherapy, when analyzing samples from patients undergoing treatment. Overall, our findings demonstrated a novel PD-L1 regulatory mechanism by WTAP-induced mRNA epigenetic regulation and the possible application of targeting WTAP as immunotherapy for tumor hypoxia.
中文翻译:

WTAP 诱导的 PD-L1 N6-甲基腺苷在结直肠癌缺氧条件下阻断 T 细胞介导的抗肿瘤活性
N 6 -甲基腺苷(m 6 A)是转录后调节基因表达的重要过程。程序性死亡配体 1 (PD-L1) 是一种主要的免疫抑制检查点,可促进免疫逃避并在肿瘤细胞中表达。在这项研究中,我们发现,在缺氧条件下,癌细胞(结直肠癌,CRC)中泛素介导的裂解引起的 Wilms 肿瘤 1 相关蛋白(WTAP)降解受到直接与 WTAP 结合的 Pumilio 同源物 1(PUM1)的抑制。 WTAP 以 m 6 A 依赖性方式增强 PD-L1 表达。 m 6 “阅读器”胰岛素样生长因子 2 mRNA 结合蛋白 2 (IGF2BP2) 识别出甲基化的 PD-L1 转录物,并随后固定其 mRNA。此外,我们发现 WTAP 在体外和体内的过度表达可以阻止 T 细胞增殖及其癌细胞杀伤作用。过度表达通过维持 PD-L1 的表达来阻止 T 细胞增殖并杀死 CRC。在人类结直肠癌和类器官组织中发现了支持 WTAP-PD-L1 调节轴的进一步证据。在分析接受治疗的患者样本时,WTAP 水平高的肿瘤似乎对抗 PD1 免疫疗法更敏感。总体而言,我们的研究结果证明了 WTAP 诱导的 mRNA 表观遗传调控的新型 PD-L1 调控机制,以及靶向 WTAP 作为肿瘤缺氧免疫疗法的可能应用。
更新日期:2024-03-21
中文翻译:

WTAP 诱导的 PD-L1 N6-甲基腺苷在结直肠癌缺氧条件下阻断 T 细胞介导的抗肿瘤活性
N 6 -甲基腺苷(m 6 A)是转录后调节基因表达的重要过程。程序性死亡配体 1 (PD-L1) 是一种主要的免疫抑制检查点,可促进免疫逃避并在肿瘤细胞中表达。在这项研究中,我们发现,在缺氧条件下,癌细胞(结直肠癌,CRC)中泛素介导的裂解引起的 Wilms 肿瘤 1 相关蛋白(WTAP)降解受到直接与 WTAP 结合的 Pumilio 同源物 1(PUM1)的抑制。 WTAP 以 m 6 A 依赖性方式增强 PD-L1 表达。 m 6 “阅读器”胰岛素样生长因子 2 mRNA 结合蛋白 2 (IGF2BP2) 识别出甲基化的 PD-L1 转录物,并随后固定其 mRNA。此外,我们发现 WTAP 在体外和体内的过度表达可以阻止 T 细胞增殖及其癌细胞杀伤作用。过度表达通过维持 PD-L1 的表达来阻止 T 细胞增殖并杀死 CRC。在人类结直肠癌和类器官组织中发现了支持 WTAP-PD-L1 调节轴的进一步证据。在分析接受治疗的患者样本时,WTAP 水平高的肿瘤似乎对抗 PD1 免疫疗法更敏感。总体而言,我们的研究结果证明了 WTAP 诱导的 mRNA 表观遗传调控的新型 PD-L1 调控机制,以及靶向 WTAP 作为肿瘤缺氧免疫疗法的可能应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号