当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement, Modeling, and Solvent Effect of 4,4′-Dimethoxybenzophenone in Ten Monosolvents and in Three Binary Solvent Mixtures from 293.15 to 333.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-21 , DOI: 10.1021/acs.jced.4c00012 Yuxiang Liu 1 , Hao Wu 1 , Han Yue 1 , Xiwei Hu 1 , Qunsheng Li 1 , Hongkang Zhao 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-21 , DOI: 10.1021/acs.jced.4c00012 Yuxiang Liu 1 , Hao Wu 1 , Han Yue 1 , Xiwei Hu 1 , Qunsheng Li 1 , Hongkang Zhao 1
Affiliation
|
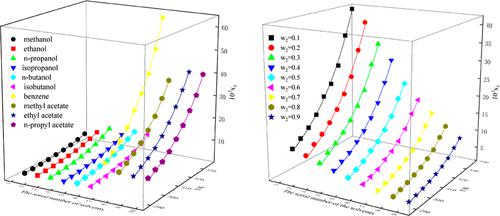
We have determined the solubility of 4,4′-dimethoxybenzophenone (DMBP) in ten monosolvents and three binary solvent mixtures at temperatures ranging from 293.15 to 333.15 K and at atmospheric pressure. The solubility of DMBP in both monosolvents and binary solvent mixtures increases with temperature. The solubility of DMBP is greatest in the solvent benzene and lowest in the solvent methanol. The Apelblat equation is the most suitable equation for DMBP in both monosolvents and binary solvent mixtures, based on the results of our solubility experiment data and the data calculated by the thermodynamic models. The outcomes of the KAT-LSER model indicate that π* and δH play a prominent role in the dissolution behavior of DMBP. The mixing thermodynamic properties (ΔmixG, ΔmixH, and ΔmixS) demonstrate that the mixing process of DMBP is an entropy-increasing and spontaneous process, and whether the process absorbs heat or not is related to the properties of the solvents.
中文翻译:

293.15 至 333.15 K 下 4,4'-二甲氧基二苯甲酮在十种单溶剂和三种二元溶剂混合物中的溶解度测量、建模和溶剂效应
我们在 293.15 至 333.15 K 的温度和大气压下测定了 4,4'-二甲氧基二苯甲酮 (DMBP) 在十种单溶剂和三种二元溶剂混合物中的溶解度。 DMBP 在单溶剂和二元溶剂混合物中的溶解度随着温度的升高而增加。 DMBP在溶剂苯中的溶解度最大,在溶剂甲醇中溶解度最低。根据我们的溶解度实验数据和热力学模型计算的数据的结果,Apelblat 方程是 DMBP 在单溶剂和二元溶剂混合物中最合适的方程。 KAT-LSER 模型的结果表明 π* 和 δ H在 DMBP 的溶出行为中发挥着重要作用。混合热力学性质(Δ mix G、Δ mix H和 Δ mix S)表明 DMBP 的混合过程是一个熵增的自发过程,该过程是否吸热与溶剂的性质有关。
更新日期:2024-03-21
中文翻译:

293.15 至 333.15 K 下 4,4'-二甲氧基二苯甲酮在十种单溶剂和三种二元溶剂混合物中的溶解度测量、建模和溶剂效应
我们在 293.15 至 333.15 K 的温度和大气压下测定了 4,4'-二甲氧基二苯甲酮 (DMBP) 在十种单溶剂和三种二元溶剂混合物中的溶解度。 DMBP 在单溶剂和二元溶剂混合物中的溶解度随着温度的升高而增加。 DMBP在溶剂苯中的溶解度最大,在溶剂甲醇中溶解度最低。根据我们的溶解度实验数据和热力学模型计算的数据的结果,Apelblat 方程是 DMBP 在单溶剂和二元溶剂混合物中最合适的方程。 KAT-LSER 模型的结果表明 π* 和 δ H在 DMBP 的溶出行为中发挥着重要作用。混合热力学性质(Δ mix G、Δ mix H和 Δ mix S)表明 DMBP 的混合过程是一个熵增的自发过程,该过程是否吸热与溶剂的性质有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号