当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Augmenting Cancer Therapy with a Supramolecular Immunogenic Cell Death Inducer: A Lysosome-Targeted NIR-Light-Activated Ruthenium(II) Metallacycle
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c13224 Le Tu 1 , Chonglu Li 1, 2 , Qihang Ding 3 , Amit Sharma 4 , Meiqin Li 1 , Junrong Li 1 , Jong Seung Kim 3 , Yao Sun 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c13224 Le Tu 1 , Chonglu Li 1, 2 , Qihang Ding 3 , Amit Sharma 4 , Meiqin Li 1 , Junrong Li 1 , Jong Seung Kim 3 , Yao Sun 1
Affiliation
|
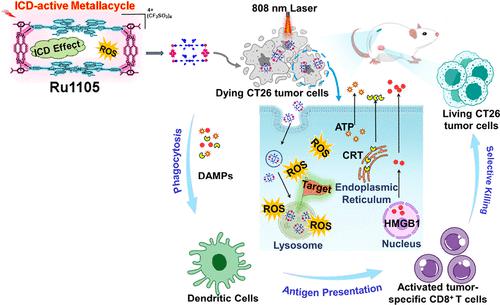
Though immunogenic cell death (ICD) has garnered significant attention in the realm of anticancer therapies, effectively stimulating strong immune responses with minimal side effects in deep-seated tumors remains challenging. Herein, we introduce a novel self-assembled near-infrared-light-activated ruthenium(II) metallacycle, Ru1105 (λem = 1105 nm), as a first example of a Ru(II) supramolecular ICD inducer. Ru1105 synergistically potentiates immunomodulatory responses and reduces adverse effects in deep-seated tumors through multiple regulated approaches, including NIR-light excitation, increased reactive oxygen species (ROS) generation, selective targeting of tumor cells, precision organelle localization, and improved tumor penetration/retention capabilities. Specifically, Ru1105 demonstrates excellent depth-activated ROS production (∼1 cm), strong resistance to diffusion, and anti-ROS quenching. Moreover, Ru1105 exhibits promising results in cellular uptake and ROS generation in cancer cells and multicellular tumor spheroids. Importantly, Ru1105 induces more efficient ICD in an ultralow dose (10 μM) compared to the conventional anticancer agent, oxaliplatin (300 μM). In vivo experiments further confirm Ru1105’s potency as an ICD inducer, eliciting CD8+ T cell responses and depleting Foxp3+ T cells with minimal adverse effects. Our research lays the foundation for the design of secure and exceptionally potent metal-based ICD agents in immunotherapy.
中文翻译:

使用超分子免疫原性细胞死亡诱导剂增强癌症治疗:溶酶体靶向近红外光激活钌 (II) 金属环
尽管免疫原性细胞死亡(ICD)在抗癌治疗领域引起了广泛关注,但在深部肿瘤中以最小的副作用有效刺激强烈的免疫反应仍然具有挑战性。在此,我们介绍了一种新型自组装近红外光激活钌(II)金属环,Ru1105(λ em = 1105 nm),作为Ru(II)超分子ICD诱导剂的第一个例子。Ru1105通过多种调节方法协同增强免疫调节反应并减少深部肿瘤的不良影响,包括近红外光激发、增加活性氧 (ROS) 生成、选择性靶向肿瘤细胞、精确细胞器定位和改善肿瘤渗透/保留能力。具体来说,Ru1105表现出优异的深度激活 ROS 产生(~1 cm)、强大的扩散阻力和抗 ROS 猝灭能力。此外,Ru1105在癌细胞和多细胞肿瘤球体中的细胞摄取和 ROS 生成方面表现出有希望的结果。重要的是,与传统抗癌剂奥沙利铂 (300 μM) 相比,Ru1105在超低剂量 (10 μM) 下诱导更有效的 ICD。体内实验进一步证实了Ru1105作为 ICD 诱导剂的效力,可引发 CD8 + T 细胞反应并耗尽 Foxp3 + T 细胞,同时将副作用降至最低。我们的研究为免疫治疗中安全且极其有效的金属基 ICD 药物的设计奠定了基础。
更新日期:2024-03-21
中文翻译:

使用超分子免疫原性细胞死亡诱导剂增强癌症治疗:溶酶体靶向近红外光激活钌 (II) 金属环
尽管免疫原性细胞死亡(ICD)在抗癌治疗领域引起了广泛关注,但在深部肿瘤中以最小的副作用有效刺激强烈的免疫反应仍然具有挑战性。在此,我们介绍了一种新型自组装近红外光激活钌(II)金属环,Ru1105(λ em = 1105 nm),作为Ru(II)超分子ICD诱导剂的第一个例子。Ru1105通过多种调节方法协同增强免疫调节反应并减少深部肿瘤的不良影响,包括近红外光激发、增加活性氧 (ROS) 生成、选择性靶向肿瘤细胞、精确细胞器定位和改善肿瘤渗透/保留能力。具体来说,Ru1105表现出优异的深度激活 ROS 产生(~1 cm)、强大的扩散阻力和抗 ROS 猝灭能力。此外,Ru1105在癌细胞和多细胞肿瘤球体中的细胞摄取和 ROS 生成方面表现出有希望的结果。重要的是,与传统抗癌剂奥沙利铂 (300 μM) 相比,Ru1105在超低剂量 (10 μM) 下诱导更有效的 ICD。体内实验进一步证实了Ru1105作为 ICD 诱导剂的效力,可引发 CD8 + T 细胞反应并耗尽 Foxp3 + T 细胞,同时将副作用降至最低。我们的研究为免疫治疗中安全且极其有效的金属基 ICD 药物的设计奠定了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号