当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optical Control of G-Actin with a Photoswitchable Latrunculin
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c10776 Nynke A. Vepřek 1, 2 , Madeline H. Cooper 3 , Laura Laprell 4 , Emily Jie-Ning Yang 5 , Sander Folkerts 1 , Ruiyang Bao 1 , Malgorzata Boczkowska 6 , Nicholas J. Palmer 6 , Roberto Dominguez 6 , Thomas G. Oertner 4 , Liza A. Pon 5 , J. Bradley Zuchero 3 , Dirk H. Trauner 1, 7
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c10776 Nynke A. Vepřek 1, 2 , Madeline H. Cooper 3 , Laura Laprell 4 , Emily Jie-Ning Yang 5 , Sander Folkerts 1 , Ruiyang Bao 1 , Malgorzata Boczkowska 6 , Nicholas J. Palmer 6 , Roberto Dominguez 6 , Thomas G. Oertner 4 , Liza A. Pon 5 , J. Bradley Zuchero 3 , Dirk H. Trauner 1, 7
Affiliation
|
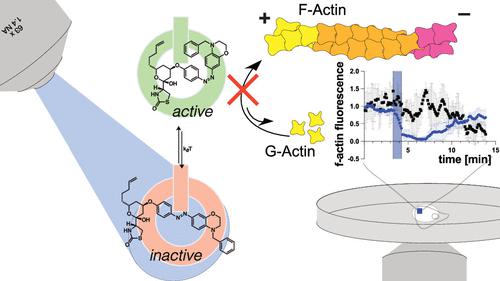
Actin is one of the most abundant proteins in eukaryotic cells and is a key component of the cytoskeleton. A range of small molecules has emerged that interfere with actin dynamics by either binding to polymeric F-actin or monomeric G-actin to stabilize or destabilize filaments or prevent their formation and growth, respectively. Among these, the latrunculins, which bind to G-actin and affect polymerization, are widely used as tools to investigate actin-dependent cellular processes. Here, we report a photoswitchable version of latrunculin, termed opto-latrunculin (OptoLat), which binds to G-actin in a light-dependent fashion and affords optical control over actin polymerization. OptoLat can be activated with 390–490 nm pulsed light and rapidly relaxes to its inactive form in the dark. Light activated OptoLat induced depolymerization of F-actin networks in oligodendrocytes and budding yeast, as shown by fluorescence microscopy. Subcellular control of actin dynamics in human cancer cell lines was demonstrated via live cell imaging. Light-activated OptoLat also reduced microglia surveillance in organotypic mouse brain slices while ramification was not affected. Incubation in the dark did not alter the structural and functional integrity of the microglia. Together, our data demonstrate that OptoLat is a useful tool for the elucidation of G-actin dependent dynamic processes in cells and tissues.
中文翻译:

用光开关 Latrunculin 对 G-肌动蛋白进行光学控制
肌动蛋白是真核细胞中最丰富的蛋白质之一,是细胞骨架的关键组成部分。已经出现了一系列小分子,它们通过与聚合的 F-肌动蛋白或单体的 G-肌动蛋白结合来分别稳定或破坏肌丝的稳定或阻止其形成和生长,从而干扰肌动蛋白动力学。其中,latrunculins 与 G-肌动蛋白结合并影响聚合,被广泛用作研究肌动蛋白依赖性细胞过程的工具。在这里,我们报道了一种可光切换的 latrunculin 版本,称为 opto-latrunculin ( OptoLat ),它以光依赖性方式与 G-肌动蛋白结合,并对肌动蛋白聚合提供光学控制。OptoLat可以用 390-490 nm 脉冲光激活,并在黑暗中迅速放松至非激活状态。荧光显微镜显示,光激活的OptoLat诱导少突胶质细胞和芽殖酵母中 F-肌动蛋白网络解聚。通过活细胞成像证明了人类癌细胞系中肌动蛋白动力学的亚细胞控制。光激活OptoLat还减少了器官型小鼠脑切片中小胶质细胞的监视,而分支不受影响。在黑暗中孵育不会改变小胶质细胞的结构和功能完整性。总之,我们的数据表明OptoLat是阐明细胞和组织中 G 肌动蛋白依赖性动态过程的有用工具。
更新日期:2024-03-21
中文翻译:

用光开关 Latrunculin 对 G-肌动蛋白进行光学控制
肌动蛋白是真核细胞中最丰富的蛋白质之一,是细胞骨架的关键组成部分。已经出现了一系列小分子,它们通过与聚合的 F-肌动蛋白或单体的 G-肌动蛋白结合来分别稳定或破坏肌丝的稳定或阻止其形成和生长,从而干扰肌动蛋白动力学。其中,latrunculins 与 G-肌动蛋白结合并影响聚合,被广泛用作研究肌动蛋白依赖性细胞过程的工具。在这里,我们报道了一种可光切换的 latrunculin 版本,称为 opto-latrunculin ( OptoLat ),它以光依赖性方式与 G-肌动蛋白结合,并对肌动蛋白聚合提供光学控制。OptoLat可以用 390-490 nm 脉冲光激活,并在黑暗中迅速放松至非激活状态。荧光显微镜显示,光激活的OptoLat诱导少突胶质细胞和芽殖酵母中 F-肌动蛋白网络解聚。通过活细胞成像证明了人类癌细胞系中肌动蛋白动力学的亚细胞控制。光激活OptoLat还减少了器官型小鼠脑切片中小胶质细胞的监视,而分支不受影响。在黑暗中孵育不会改变小胶质细胞的结构和功能完整性。总之,我们的数据表明OptoLat是阐明细胞和组织中 G 肌动蛋白依赖性动态过程的有用工具。



























 京公网安备 11010802027423号
京公网安备 11010802027423号