当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereochemical and Computational NMR Survey of 1,2,3-Triazoles: in Search of the Original Tauto-Conformers
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-21 , DOI: 10.1021/acs.jpca.3c08217 Valentin A. Semenov 1 , Lyudmila I. Larina 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-21 , DOI: 10.1021/acs.jpca.3c08217 Valentin A. Semenov 1 , Lyudmila I. Larina 1
Affiliation
|
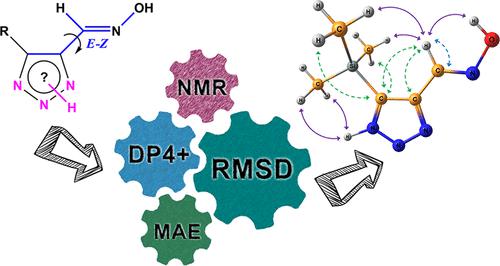
The conformational analysis of nine functionalized 1,2,3-triazoles was carried out by the correlation of calculated and experimental high-level nuclear magnetic resonance (NMR) chemical shifts. In solution, the studied triazoles are in exchange dynamic equilibrium caused by their prototropic tautomerism of the NH-proton. The experimentally unresolved NMR signals were assigned for most of the compounds. A more thorough survey was conducted for 4-t-butyl-1,2,3-triazole-5-carbaldehyde oxime. The analysis performed within the framework of the DP4+ formalism completely confirmed the hypothesis of the predominance of the 2H-tautomer. Thus, the methodology for estimating stereochemical structures in the absence of some experimental data allowed the most stable conformations for dynamic systems with different tautomeric ratios to be reliably identified.
中文翻译:

1,2,3-三唑的立体化学和计算核磁共振研究:寻找原始的 Tauto 构象异构体
通过计算和实验高水平核磁共振 (NMR) 化学位移的相关性,对九种官能化 1,2,3-三唑进行构象分析。在溶液中,所研究的三唑由于 NH-质子的质子互变异构而处于交换动态平衡。大多数化合物都归属于实验上未解析的 NMR 信号。对 4-叔丁基-1,2,3-三唑-5-甲醛肟进行了更彻底的调查。在 DP4+ 形式主义框架内进行的分析完全证实了 2H-互变异构体占主导地位的假设。因此,在缺乏一些实验数据的情况下估计立体化学结构的方法可以可靠地识别具有不同互变异构比率的动态系统的最稳定构象。
更新日期:2024-03-22
中文翻译:

1,2,3-三唑的立体化学和计算核磁共振研究:寻找原始的 Tauto 构象异构体
通过计算和实验高水平核磁共振 (NMR) 化学位移的相关性,对九种官能化 1,2,3-三唑进行构象分析。在溶液中,所研究的三唑由于 NH-质子的质子互变异构而处于交换动态平衡。大多数化合物都归属于实验上未解析的 NMR 信号。对 4-叔丁基-1,2,3-三唑-5-甲醛肟进行了更彻底的调查。在 DP4+ 形式主义框架内进行的分析完全证实了 2H-互变异构体占主导地位的假设。因此,在缺乏一些实验数据的情况下估计立体化学结构的方法可以可靠地识别具有不同互变异构比率的动态系统的最稳定构象。



























 京公网安备 11010802027423号
京公网安备 11010802027423号