Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Study of dual binding specificity of aptamer to ochratoxin A and norfloxacin and the development of fluorescent aptasensor in milk detection
Talanta ( IF 6.1 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.talanta.2024.125935 Ling Gao , Yue Zhang , Lu Chen , Qingtong Zhou , Nandi Zhou , Xiaole Xia
Talanta ( IF 6.1 ) Pub Date : 2024-03-19 , DOI: 10.1016/j.talanta.2024.125935 Ling Gao , Yue Zhang , Lu Chen , Qingtong Zhou , Nandi Zhou , Xiaole Xia
|
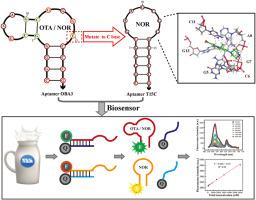
Target specificity, one of aptamer characteristics that determine recognition efficiency of biosensors, is generally considered to be an intrinsic property of aptamer. However, a high-affinity aptamer may have additional target binding specificity, little is known about the specificity of aptamer binding to multiple targets, which may result in false-positive results that hinder the accuracy of detection. Herein, an aptamer OBA3 with dual target ochratoxin A (OTA) and norfloxacin (NOR) was used as an example to explore the binding specificity mechanism and developed rapid fluorescent aptasensing methods. The nucleotide 15th T of aptamer OBA3 was demonstrated to be critical for specificity and affinity binding of target OTA via site-saturation mutagenesis. Substituting the 15th T base for C base could directly improve recognition specificity of aptamer for NOR and remove the binding affinity for OTA. The combination of π-π stacking interactions, salt bridges and hydrogen bonds between loop pocket of aptamer and quinolone skeleton, piperazinyl group may contributes to the fluoroquinolone antibiotics (NOR and difloxacin)-aptamer recognition interaction. Based on this understanding, a dual-aptamer fluorescent biosensor was fabricated for simultaneous detection of OTA and NOR, which has a linear detection range of 50–6000 nM with a detection limit of 31 nM for OTA and NOR. Combined with T15C biosensor for eliminating interference of OTA, the assay was applied to milk samples with satisfactory recovery (94.06–100.93%), which can achieve detection of OTA and NOR individually within 40 min.
中文翻译:

适体对赭曲霉毒素A和诺氟沙星双重结合特异性的研究及牛奶检测荧光适体传感器的研制
目标特异性是决定生物传感器识别效率的适体特征之一,通常被认为是适体的固有属性。然而,高亲和力的适配体可能具有额外的靶点结合特异性,人们对适配体与多个靶点结合的特异性知之甚少,这可能会导致假阳性结果,影响检测的准确性。在此,以具有双靶点赭曲霉毒素A(OTA)和诺氟沙星(NOR)的适配体OBA3为例,探讨了结合特异性机制并开发了快速荧光适配体传感方法。适配体 OBA3 的第 15 个 T 核苷酸被证明对于通过位点饱和诱变实现靶标 OTA 的特异性和亲和力结合至关重要。将第15位T碱基替换为C碱基可以直接提高适配体对NOR的识别特异性并消除对OTA的结合亲和力。适配体环袋与喹诺酮骨架、哌嗪基之间的π-π堆积相互作用、盐桥和氢键的组合可能有助于氟喹诺酮类抗生素(NOR和二氟沙星)-适配体识别相互作用。基于这一认识,我们制备了一种双适体荧光生物传感器,用于同时检测OTA和NOR,其线性检测范围为50-6000 nM,OTA和NOR的检测限为31 nM。结合消除OTA干扰的T15C生物传感器,该方法应用于牛奶样品,回收率令人满意(94.06-100.93%),可在40 min内实现OTA和NOR的单独检测。
更新日期:2024-03-19
中文翻译:

适体对赭曲霉毒素A和诺氟沙星双重结合特异性的研究及牛奶检测荧光适体传感器的研制
目标特异性是决定生物传感器识别效率的适体特征之一,通常被认为是适体的固有属性。然而,高亲和力的适配体可能具有额外的靶点结合特异性,人们对适配体与多个靶点结合的特异性知之甚少,这可能会导致假阳性结果,影响检测的准确性。在此,以具有双靶点赭曲霉毒素A(OTA)和诺氟沙星(NOR)的适配体OBA3为例,探讨了结合特异性机制并开发了快速荧光适配体传感方法。适配体 OBA3 的第 15 个 T 核苷酸被证明对于通过位点饱和诱变实现靶标 OTA 的特异性和亲和力结合至关重要。将第15位T碱基替换为C碱基可以直接提高适配体对NOR的识别特异性并消除对OTA的结合亲和力。适配体环袋与喹诺酮骨架、哌嗪基之间的π-π堆积相互作用、盐桥和氢键的组合可能有助于氟喹诺酮类抗生素(NOR和二氟沙星)-适配体识别相互作用。基于这一认识,我们制备了一种双适体荧光生物传感器,用于同时检测OTA和NOR,其线性检测范围为50-6000 nM,OTA和NOR的检测限为31 nM。结合消除OTA干扰的T15C生物传感器,该方法应用于牛奶样品,回收率令人满意(94.06-100.93%),可在40 min内实现OTA和NOR的单独检测。



























 京公网安备 11010802027423号
京公网安备 11010802027423号