Structure ( IF 5.7 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.str.2024.02.013 Daniel Wohlwend , Luca Mérono , Sarah Bucka , Kevin Ritter , Henning J. Jessen , Thorsten Friedrich
|
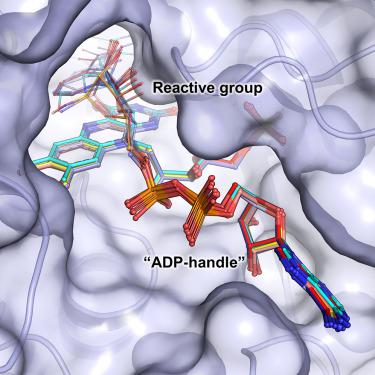
Energy-converting NADH:ubiquinone oxidoreductase, respiratory complex I, is a major enzyme of energy metabolism that couples NADH oxidation and ubiquinone reduction with proton translocation. The NADH oxidation site features different enzymatic activities with various nucleotides. While the kinetics of these reactions are well described, only binding of NAD+ and NADH have been structurally characterized. Here, we report the structures of the electron input module of Aquifex aeolicus complex I with bound ADP-ribose and 3-acetylpyridine adenine dinucleotides at resolutions better than 2.0 Å. ADP-ribose acts as inhibitor by blocking the “ADP-handle” motif essential for nucleotide binding. The pyridine group of APADH is minimally offset from flavin, which could contribute to its poorer suitability as substrate. A comparison with other nucleotide co-structures surprisingly shows that the adenine ribose and the pyrophosphate moiety contribute most to nucleotide binding, thus all adenine dinucleotides share core binding modes to the unique Rossmann-fold in complex I.
中文翻译:

与呼吸复合物 I 电子输入模块结合的 3-乙酰吡啶腺嘌呤二核苷酸和 ADP-核糖的结构
能量转换 NADH:泛醌氧化还原酶,呼吸复合物 I,是能量代谢的主要酶,将 NADH 氧化和泛醌还原与质子易位结合起来。 NADH 氧化位点对不同的核苷酸具有不同的酶活性。虽然这些反应的动力学已得到很好的描述,但仅对 NAD +和 NADH 的结合进行了结构表征。在这里,我们报告了Aquifex aeolicus复合物 I的电子输入模块的结构,该模块结合了 ADP-核糖和 3-乙酰吡啶腺嘌呤二核苷酸,分辨率优于 2.0 Å。 ADP-核糖通过阻断核苷酸结合所必需的“ADP-handle”基序来充当抑制剂。 APADH 的吡啶基团与黄素的偏移极小,这可能导致其作为底物的适用性较差。与其他核苷酸共结构的比较令人惊讶地表明,腺嘌呤核糖和焦磷酸部分对核苷酸结合贡献最大,因此所有腺嘌呤二核苷酸共享与复合物I中独特的罗斯曼折叠的核心结合模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号