当前位置:
X-MOL 学术
›
Mol. Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Immune checkpoint inhibitor monotherapy is sufficient to promote microenvironmental normalization via the type I interferon pathway in PD‐L1‐expressing head and neck cancer
Molecular Oncology ( IF 6.6 ) Pub Date : 2024-03-21 , DOI: 10.1002/1878-0261.13633 Jeon Yeob Jang 1, 2 , Bok‐Soon Lee 1 , Mei Huang 1 , Chorong Seo 1 , Ji‐Hye Choi 3 , Yoo Seob Shin 1 , Hyun Goo Woo 3 , Chul‐Ho Kim 1, 4
Molecular Oncology ( IF 6.6 ) Pub Date : 2024-03-21 , DOI: 10.1002/1878-0261.13633 Jeon Yeob Jang 1, 2 , Bok‐Soon Lee 1 , Mei Huang 1 , Chorong Seo 1 , Ji‐Hye Choi 3 , Yoo Seob Shin 1 , Hyun Goo Woo 3 , Chul‐Ho Kim 1, 4
Affiliation
|
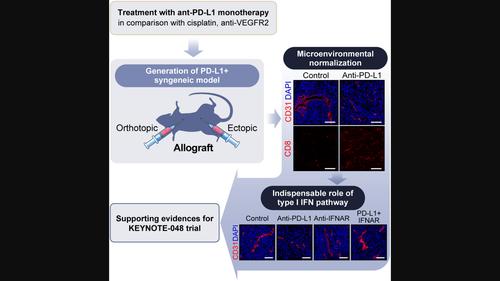
Immune checkpoint blockers (ICBs) targeting programmed cell death protein 1 (PD‐1) have been proven to be an effective first‐line therapy against programmed cell death 1 ligand 1 (PD‐L1 ; also known as CD274 molecule)‐expressing head and neck squamous cell carcinoma (HNSCC) in recent KEYNOTE‐048 trial. However, associated changes in the tumor microenvironment (TME) and underlying mechanisms remain elusive. Oral tumors in C57/BL6 mice were induced by administering 7,12‐dimethylbenzanthracene into the buccal mucosa. Single‐cell suspension was isolated from tumor tissue; proliferating cells were injected subcutaneously into the left flank of mice to establish Ajou oral cancer (AOC) cell lines. Subsequently, a syngeneic PD‐L1 ‐expressing HNSCC model was developed by injecting AOC cells into the buccal or tongue area. The model recapitulated human HNSCC molecular features and showed reliable in vivo tumorigenicity with significant PD‐L1 expression. ICB monotherapy induced global changes in the TME, including vascular normalization. Furthermore, the antitumor effect of ICB monotherapy was superior to those of other therapeutic agents, including cisplatin and inhibitors of vascular endothelial growth factor receptor 2 (VEGFR2). The ICB‐induced antitumorigenicity and TME normalization were alleviated by blocking the type I interferon pathway. In summary, ICB monotherapy is sufficient to induce TME normalization in the syngeneic model; the type I interferon pathway is indispensable in realizing the effects of ICBs. Furthermore, these results explain the underlying mechanism of the efficacy of ICB monotherapy against PD‐L1 ‐expressing HNSCC in the KEYNOTE‐048 trial.
中文翻译:

免疫检查点抑制剂单一疗法足以通过 I 型干扰素途径促进表达 PD-L1 的头颈癌微环境正常化
靶向程序性细胞死亡蛋白 1 (PD-1) 的免疫检查点阻断剂 (ICB) 已被证明是针对程序性细胞死亡 1 配体 1 的有效一线疗法(PD-L1 ;最近的 KEYNOTE-048 试验中,也称为 CD274 分子)表达头颈鳞状细胞癌 (HNSCC)。然而,肿瘤微环境(TME)的相关变化和潜在机制仍然难以捉摸。通过将 7,12-二甲基苯并蒽注入颊粘膜来诱导 C57/BL6 小鼠口腔肿瘤。从肿瘤组织中分离出单细胞悬液;将增殖细胞皮下注射到小鼠左侧,建立 Ajou 口腔癌 (AOC) 细胞系。随后,同系PD-L1 ‐表达 HNSCC 模型是通过将 AOC 细胞注射到颊或舌区域而开发的。该模型重现了人类 HNSCC 的分子特征,并显示出可靠的结果体内 具有显着的致瘤性PD-L1 表达。 ICB 单一疗法引起 TME 的整体变化,包括血管正常化。此外,ICB单一疗法的抗肿瘤效果优于其他治疗药物,包括顺铂和血管内皮生长因子受体2(VEGFR2)抑制剂。 ICB 诱导的抗肿瘤作用和 TME 正常化通过阻断 I 型干扰素途径得到缓解。总之,ICB单一疗法足以诱导同基因模型中的TME正常化; I型干扰素途径对于实现ICB的作用是不可或缺的。此外,这些结果解释了 ICB 单一疗法针对癌症的疗效的潜在机制。PD-L1 ‐在 KEYNOTE‐048 试验中表达 HNSCC。
更新日期:2024-03-21
中文翻译:

免疫检查点抑制剂单一疗法足以通过 I 型干扰素途径促进表达 PD-L1 的头颈癌微环境正常化
靶向程序性细胞死亡蛋白 1 (PD-1) 的免疫检查点阻断剂 (ICB) 已被证明是针对程序性细胞死亡 1 配体 1 的有效一线疗法(



























 京公网安备 11010802027423号
京公网安备 11010802027423号