当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Atroposelective C–H Alkylation Enabled by Bimetallic Catalysis with Air-Stable Heteroatom-Substituted Secondary Phosphine Oxide Preligands
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c14600 Zi-Jing Zhang 1 , Matthias M. Simon 1 , Shuang Yu 2 , Shu-Wen Li 2 , Xinran Chen 1 , Silvia Cattani 1 , Xin Hong 2 , Lutz Ackermann 1, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c14600 Zi-Jing Zhang 1 , Matthias M. Simon 1 , Shuang Yu 2 , Shu-Wen Li 2 , Xinran Chen 1 , Silvia Cattani 1 , Xin Hong 2 , Lutz Ackermann 1, 3
Affiliation
|
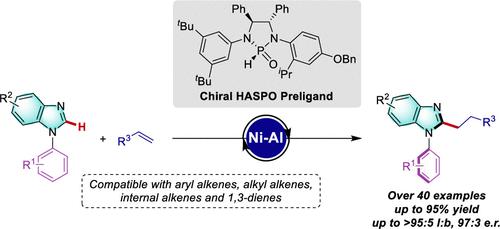
The catalytic asymmetric construction of axially chiral C–N atropisomers remains a formidable challenge due to their low rotational barriers and is largely reliant on toxic, cost-intensive, and precious metal catalysts. In sharp contrast, we herein describe the first nickel-catalyzed atroposelective C–H alkylation for the construction of C–N axially chiral compounds with the aid of a chiral heteroatom-substituted secondary phosphine oxide (HASPO)-ligated Ni–Al bimetallic catalyst. A wide range of alkenes, including terminal and internal alkenes, were well compatible with the reaction, providing a variety of benzimidazole derivatives in high yields and enantioselectivities (up to 97:3 e.r.). The key to success was the identification of novel HASPOs as highly effective chiral preligands. Mechanistic studies revealed the catalyst mode of action, and in-depth data science analysis elucidated the key features of the responsible chiral preligands in controlling the enantioselectivity.
中文翻译:

空气稳定的杂原子取代的二级氧化膦预配体通过双金属催化实现镍催化的对位选择性 C–H 烷基化
由于旋转势垒较低,轴向手性 C-N 阻转异构体的催化不对称结构仍然是一个艰巨的挑战,并且在很大程度上依赖于有毒、成本密集的贵金属催化剂。与此形成鲜明对比的是,我们在此描述了第一个镍催化的原子选择性C-H烷基化反应,借助手性杂原子取代的二氧化膦(HASPO)配位的Ni-Al双金属催化剂来构建C-N轴向手性化合物。多种烯烃,包括末端烯烃和内部烯烃,与反应良好相容,以高产率和对映选择性(高达 97:3 er)提供多种苯并咪唑衍生物。成功的关键是将新型 HASPO 鉴定为高效的手性预配体。机理研究揭示了催化剂的作用模式,深入的数据科学分析阐明了负责控制对映选择性的手性预配体的关键特征。
更新日期:2024-03-21
中文翻译:

空气稳定的杂原子取代的二级氧化膦预配体通过双金属催化实现镍催化的对位选择性 C–H 烷基化
由于旋转势垒较低,轴向手性 C-N 阻转异构体的催化不对称结构仍然是一个艰巨的挑战,并且在很大程度上依赖于有毒、成本密集的贵金属催化剂。与此形成鲜明对比的是,我们在此描述了第一个镍催化的原子选择性C-H烷基化反应,借助手性杂原子取代的二氧化膦(HASPO)配位的Ni-Al双金属催化剂来构建C-N轴向手性化合物。多种烯烃,包括末端烯烃和内部烯烃,与反应良好相容,以高产率和对映选择性(高达 97:3 er)提供多种苯并咪唑衍生物。成功的关键是将新型 HASPO 鉴定为高效的手性预配体。机理研究揭示了催化剂的作用模式,深入的数据科学分析阐明了负责控制对映选择性的手性预配体的关键特征。



























 京公网安备 11010802027423号
京公网安备 11010802027423号