当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
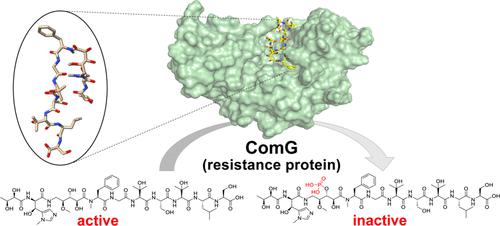
The Peptide Antibiotic Corramycin Adopts a β-Hairpin-like Structure and Is Inactivated by the Kinase ComG
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c13208 Sebastian Adam 1 , Franziska Fries 1, 2, 3 , Alexander von Tesmar 1 , Sari Rasheed 1, 3 , Selina Deckarm 1 , Carla F. Sousa 1 , Roman Reberšek 1 , Timo Risch 1, 2 , Stefano Mancini 4 , Jennifer Herrmann 1, 3 , Jesko Koehnke 1, 5 , Olga V. Kalinina 1, 6, 7 , Rolf Müller 1, 2, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-21 , DOI: 10.1021/jacs.3c13208 Sebastian Adam 1 , Franziska Fries 1, 2, 3 , Alexander von Tesmar 1 , Sari Rasheed 1, 3 , Selina Deckarm 1 , Carla F. Sousa 1 , Roman Reberšek 1 , Timo Risch 1, 2 , Stefano Mancini 4 , Jennifer Herrmann 1, 3 , Jesko Koehnke 1, 5 , Olga V. Kalinina 1, 6, 7 , Rolf Müller 1, 2, 3
Affiliation
|
The rapid development of antibiotic resistance, especially among difficult-to-treat Gram-negative bacteria, is recognized as a serious and urgent threat to public health. The detection and characterization of novel resistance mechanisms are essential to better predict the spread and evolution of antibiotic resistance. Corramycin is a novel and modified peptidic antibiotic with activity against several Gram-negative pathogens. We demonstrate that the kinase ComG, part of the corramycin biosynthetic gene cluster, phosphorylates and thereby inactivates corramycin, leading to the resistance of the host. Remarkably, we found that the closest structural homologues of ComG are aminoglycoside phosphotransferases; however, ComG shows no activity toward this class of antibiotics. The crystal structure of ComG in complex with corramycin reveals that corramycin adopts a β-hairpin-like structure and allowed us to define the changes leading to a switch in substrate from sugar to peptide. Bioinformatic analyses suggest a limited occurrence of ComG-like proteins, which along with the absence of cross-resistance to clinically used drugs positions corramycin as an attractive antibiotic for further development.
中文翻译:

肽类抗生素 Corramycin 采用 β-发夹样结构并被激酶 ComG 灭活
抗生素耐药性的迅速发展,特别是难以治疗的革兰氏阴性细菌,被认为是对公众健康的严重而紧迫的威胁。新型耐药机制的检测和表征对于更好地预测抗生素耐药性的传播和演变至关重要。 Corramycin 是一种新型改良肽类抗生素,具有对抗多种革兰氏阴性病原体的活性。我们证明激酶 ComG(科拉霉素生物合成基因簇的一部分)会磷酸化,从而使科拉霉素失活,导致宿主产生耐药性。值得注意的是,我们发现 ComG 最接近的结构同源物是氨基糖苷类磷酸转移酶;然而,ComG 对此类抗生素没有显示出活性。 ComG 与 Corramycin 复合物的晶体结构表明 Corramycin 采用 β-发夹状结构,使我们能够定义导致底物从糖转换为肽的变化。生物信息学分析表明,ComG 样蛋白的出现有限,并且与临床使用的药物不存在交叉耐药性,使得考拉霉素成为一种有吸引力的抗生素,有待进一步开发。
更新日期:2024-03-21
中文翻译:

肽类抗生素 Corramycin 采用 β-发夹样结构并被激酶 ComG 灭活
抗生素耐药性的迅速发展,特别是难以治疗的革兰氏阴性细菌,被认为是对公众健康的严重而紧迫的威胁。新型耐药机制的检测和表征对于更好地预测抗生素耐药性的传播和演变至关重要。 Corramycin 是一种新型改良肽类抗生素,具有对抗多种革兰氏阴性病原体的活性。我们证明激酶 ComG(科拉霉素生物合成基因簇的一部分)会磷酸化,从而使科拉霉素失活,导致宿主产生耐药性。值得注意的是,我们发现 ComG 最接近的结构同源物是氨基糖苷类磷酸转移酶;然而,ComG 对此类抗生素没有显示出活性。 ComG 与 Corramycin 复合物的晶体结构表明 Corramycin 采用 β-发夹状结构,使我们能够定义导致底物从糖转换为肽的变化。生物信息学分析表明,ComG 样蛋白的出现有限,并且与临床使用的药物不存在交叉耐药性,使得考拉霉素成为一种有吸引力的抗生素,有待进一步开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号