当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis Landscapes for Ammonia Borane Chemical Vapor Deposition of h-BN and BNNT: Unraveling Reactions and Intermediates from First-Principles
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-22 , DOI: 10.1021/jacs.4c01354 Ting Cheng 1 , Ksenia V. Bets 1 , Boris I. Yakobson 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-22 , DOI: 10.1021/jacs.4c01354 Ting Cheng 1 , Ksenia V. Bets 1 , Boris I. Yakobson 1, 2
Affiliation
|
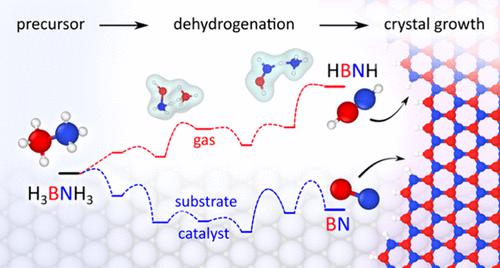
Planar hexagonal boron nitride (h-BN) and tubular BN nanotube (BNNT), known for their superior mechanical and thermal properties, as well as wide electronic band gap, hold great potential for nanoelectronic and optoelectronic devices. Chemical vapor deposition has demonstrated the best way to scalable synthesis of high-quality BN nanomaterials. Yet, the atomistic understanding of reactions from precursors to product-material remains elusive, posing challenges for experimental design. Here, performing first-principles calculations and ab initio molecular simulations, we explore pyrolytic decomposition pathways of the most used precursor ammonia borane (H3BNH3, AB) to BN, in gas-phase and on Ni(111) or amorphous boron (for BNNT growth) surfaces, for comparison. It reveals that in the gas phase, a pair of AB molecules cooperate to form intermediate NH3 and ammonia diborane, which further dissociates into H2BNH2, accompanied by critical BH4– and NH4+ ions. These ions act as H scavengers facilitating H2BNH2 dehydrogenation into HBNH. The consequent HBNH directly feeds BN flake growth by reacting with the crystal edge, while the addition of H2BNH2 to the edge is prohibited at 1500 K. In contrast, on Ni and boron surfaces, AB monomer dehydrogenates stepwise, deeper, yielding BNH and BN dimer as the primary building unit. Our study maps out three typical experimental conditions regarding the dissociation of AB-precursor, providing insights into the underlying reaction mechanisms of gas-phase precursors, to help as guidelines for the experimental growth of BN nanomaterials.
中文翻译:

h-BN 和 BNNT 的氨硼烷化学气相沉积的合成景观:从第一原理中揭示反应和中间体
平面六方氮化硼(h -BN)和管状氮化硼纳米管(BNNT)以其优异的机械和热性能以及较宽的电子带隙而闻名,在纳米电子和光电器件方面具有巨大的潜力。化学气相沉积已被证明是大规模合成高质量氮化硼纳米材料的最佳方法。然而,对从前体到产物材料的反应的原子理解仍然难以捉摸,这给实验设计带来了挑战。在这里,我们通过第一性原理计算和从头算分子模拟,探索了最常用的前体氨硼烷 (H 3 BNH 3 , AB) 在气相中以及在 Ni(111) 或无定形硼上热解分解成BN 的途径(用于 BNNT 生长)表面,用于比较。结果表明,在气相中,一对AB分子协同形成中间体NH 3和氨乙硼烷,后者进一步解离成H 2 BNH 2,并伴随着关键的BH 4 -和NH 4 +离子。这些离子充当H清除剂,促进H 2 BNH 2脱氢成HBNH。由此产生的 HBNH 通过与晶体边缘反应直接促进 BN 片状生长,而在 1500 K 时禁止向边缘添加 H 2 BNH 2 。相反,在 Ni 和硼表面上,AB 单体逐步、更深地脱氢,产生 BNH BN二聚体作为主要构建单元。我们的研究列出了关于 AB 前体解离的三种典型实验条件,为气相前体的潜在反应机制提供了见解,为 BN 纳米材料的实验生长提供了指导。
更新日期:2024-03-22
中文翻译:

h-BN 和 BNNT 的氨硼烷化学气相沉积的合成景观:从第一原理中揭示反应和中间体
平面六方氮化硼(h -BN)和管状氮化硼纳米管(BNNT)以其优异的机械和热性能以及较宽的电子带隙而闻名,在纳米电子和光电器件方面具有巨大的潜力。化学气相沉积已被证明是大规模合成高质量氮化硼纳米材料的最佳方法。然而,对从前体到产物材料的反应的原子理解仍然难以捉摸,这给实验设计带来了挑战。在这里,我们通过第一性原理计算和从头算分子模拟,探索了最常用的前体氨硼烷 (H 3 BNH 3 , AB) 在气相中以及在 Ni(111) 或无定形硼上热解分解成BN 的途径(用于 BNNT 生长)表面,用于比较。结果表明,在气相中,一对AB分子协同形成中间体NH 3和氨乙硼烷,后者进一步解离成H 2 BNH 2,并伴随着关键的BH 4 -和NH 4 +离子。这些离子充当H清除剂,促进H 2 BNH 2脱氢成HBNH。由此产生的 HBNH 通过与晶体边缘反应直接促进 BN 片状生长,而在 1500 K 时禁止向边缘添加 H 2 BNH 2 。相反,在 Ni 和硼表面上,AB 单体逐步、更深地脱氢,产生 BNH BN二聚体作为主要构建单元。我们的研究列出了关于 AB 前体解离的三种典型实验条件,为气相前体的潜在反应机制提供了见解,为 BN 纳米材料的实验生长提供了指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号