当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cooperative Effects Drive Water Oxidation Catalysis in Cobalt Electrocatalysts through the Destabilization of Intermediates
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-22 , DOI: 10.1021/jacs.3c11651 Benjamin Moss 1 , Katrine Louise Svane 2 , David Nieto-Castro 3 , Reshma R. Rao 1 , Soren B. Scott 1 , Cindy Tseng 1, 4 , Michael Sachs 5 , Anuj Pennathur 4 , Caiwu Liang 1 , Louise I. Oldham 1 , Eva Mazzolini 1 , Lole Jurado 3 , Gopinathan Sankar 5 , Stephen Parry 6 , Veronica Celorrio 6 , Jahan M. Dawlaty 4 , Jan Rossmeisl 2 , J. R. Galán-Mascarós 3, 7 , Ifan E. L. Stephens 1 , James R. Durrant 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-22 , DOI: 10.1021/jacs.3c11651 Benjamin Moss 1 , Katrine Louise Svane 2 , David Nieto-Castro 3 , Reshma R. Rao 1 , Soren B. Scott 1 , Cindy Tseng 1, 4 , Michael Sachs 5 , Anuj Pennathur 4 , Caiwu Liang 1 , Louise I. Oldham 1 , Eva Mazzolini 1 , Lole Jurado 3 , Gopinathan Sankar 5 , Stephen Parry 6 , Veronica Celorrio 6 , Jahan M. Dawlaty 4 , Jan Rossmeisl 2 , J. R. Galán-Mascarós 3, 7 , Ifan E. L. Stephens 1 , James R. Durrant 1
Affiliation
|
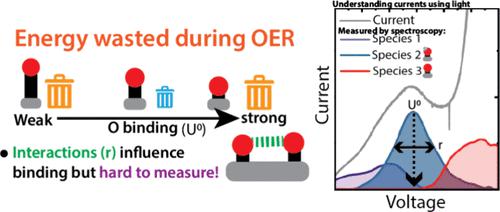
A barrier to understanding the factors driving catalysis in the oxygen evolution reaction (OER) is understanding multiple overlapping redox transitions in the OER catalysts. The complexity of these transitions obscure the relationship between the coverage of adsorbates and OER kinetics, leading to an experimental challenge in measuring activity descriptors, such as binding energies, as well as adsorbate interactions, which may destabilize intermediates and modulate their binding energies. Herein, we utilize a newly designed optical spectroelectrochemistry system to measure these phenomena in order to contrast the behavior of two electrocatalysts, cobalt oxyhydroxide (CoOOH) and cobalt–iron hexacyanoferrate (cobalt–iron Prussian blue, CoFe-PB). Three distinct optical spectra are observed in each catalyst, corresponding to three separate redox transitions, the last of which we show to be active for the OER using time-resolved spectroscopy and electrochemical mass spectroscopy. By combining predictions from density functional theory with parameters obtained from electroadsorption isotherms, we demonstrate that a destabilization of catalytic intermediates occurs with increasing coverage. In CoOOH, a strong (∼0.34 eV/monolayer) destabilization of a strongly bound catalytic intermediate is observed, leading to a potential offset between the accumulation of the intermediate and measurable O2 evolution. We contrast these data to CoFe-PB, where catalytic intermediate generation and O2 evolution onset coincide due to weaker binding and destabilization (∼0.19 eV/monolayer). By considering a correlation between activation energy and binding strength, we suggest that such adsorbate driven destabilization may account for a significant fraction of the observed OER catalytic activity in both materials. Finally, we disentangle the effects of adsorbate interactions on state coverages and kinetics to show how adsorbate interactions determine the observed Tafel slopes. Crucially, the case of CoFe-PB shows that, even where interactions are weaker, adsorption remains non-Nernstian, which strongly influences the observed Tafel slope.
中文翻译:

协同效应通过中间体的不稳定驱动钴电催化剂中的水氧化催化
了解析氧反应 (OER) 中催化作用的驱动因素的一个障碍是了解 OER 催化剂中多个重叠的氧化还原转变。这些转变的复杂性掩盖了吸附物覆盖范围和 OER 动力学之间的关系,导致测量活性描述符(例如结合能)以及吸附物相互作用面临实验挑战,这可能会破坏中间体的稳定性并调节其结合能。在这里,我们利用新设计的光学光谱电化学系统来测量这些现象,以对比两种电催化剂:羟基氧化钴(CoOOH)和六氰基铁酸钴铁(钴铁普鲁士蓝,CoFe-PB)的行为。在每种催化剂中观察到三个不同的光谱,对应于三个单独的氧化还原转变,我们使用时间分辨光谱和电化学质谱表明最后一个对 OER 具有活性。通过将密度泛函理论的预测与电吸附等温线获得的参数相结合,我们证明催化中间体的稳定性随着覆盖率的增加而发生。在CoOOH中,观察到强结合催化中间体的强烈(~0.34 eV/单层)不稳定,导致中间体的积累和可测量的O 2释放之间的潜在抵消。我们将这些数据与 CoFe-PB 进行对比,其中由于较弱的结合和不稳定(~0.19 eV/单层),催化中间体的生成和 O 2演化开始同时发生。通过考虑活化能和结合强度之间的相关性,我们认为这种吸附物驱动的不稳定可能是在两种材料中观察到的 OER 催化活性的重要组成部分。最后,我们理清了吸附质相互作用对状态覆盖和动力学的影响,以展示吸附质相互作用如何决定观察到的塔菲尔斜率。至关重要的是,CoFe-PB 的情况表明,即使相互作用较弱,吸附仍然是非能斯特的,这强烈影响观察到的塔菲尔斜率。
更新日期:2024-03-22
中文翻译:

协同效应通过中间体的不稳定驱动钴电催化剂中的水氧化催化
了解析氧反应 (OER) 中催化作用的驱动因素的一个障碍是了解 OER 催化剂中多个重叠的氧化还原转变。这些转变的复杂性掩盖了吸附物覆盖范围和 OER 动力学之间的关系,导致测量活性描述符(例如结合能)以及吸附物相互作用面临实验挑战,这可能会破坏中间体的稳定性并调节其结合能。在这里,我们利用新设计的光学光谱电化学系统来测量这些现象,以对比两种电催化剂:羟基氧化钴(CoOOH)和六氰基铁酸钴铁(钴铁普鲁士蓝,CoFe-PB)的行为。在每种催化剂中观察到三个不同的光谱,对应于三个单独的氧化还原转变,我们使用时间分辨光谱和电化学质谱表明最后一个对 OER 具有活性。通过将密度泛函理论的预测与电吸附等温线获得的参数相结合,我们证明催化中间体的稳定性随着覆盖率的增加而发生。在CoOOH中,观察到强结合催化中间体的强烈(~0.34 eV/单层)不稳定,导致中间体的积累和可测量的O 2释放之间的潜在抵消。我们将这些数据与 CoFe-PB 进行对比,其中由于较弱的结合和不稳定(~0.19 eV/单层),催化中间体的生成和 O 2演化开始同时发生。通过考虑活化能和结合强度之间的相关性,我们认为这种吸附物驱动的不稳定可能是在两种材料中观察到的 OER 催化活性的重要组成部分。最后,我们理清了吸附质相互作用对状态覆盖和动力学的影响,以展示吸附质相互作用如何决定观察到的塔菲尔斜率。至关重要的是,CoFe-PB 的情况表明,即使相互作用较弱,吸附仍然是非能斯特的,这强烈影响观察到的塔菲尔斜率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号