当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fractionation of Methane Isotopologues during Preparation for Analysis from Ambient Air
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.analchem.3c04891 Emmal Safi 1 , Tim Arnold 1, 2 , Chris Rennick 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.analchem.3c04891 Emmal Safi 1 , Tim Arnold 1, 2 , Chris Rennick 1
Affiliation
|
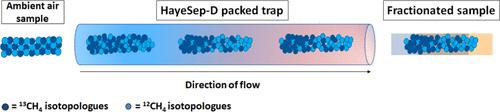
Preconcentration of methane (CH4) from air is a critical sampling step in the measurement of singly and doubly substituted isotopologue ratios. We demonstrate the potential for isotope fractionation during preconcentration onto and elution from the common trapping material HayeSep-D and investigate its significance in laser spectroscopy measurements. By altering the trapping temperature for adsorption, the flow direction of CH4 through the trap and the time at which CH4 is eluted during a desorption temperature ramp, we explain the mechanisms behind fractionation affecting δ13C(CH4) and δ2H(CH4). The results highlight that carbon isotope fractionation is driven by advection and diffusion, while hydrogen isotope fractionation is driven by the interaction of CH4 with the adsorbing material (tending to smaller isotopic effects at higher temperatures). We have compared the difference between the measured isotope ratio of sample gases (compressed whole air and a synthetic mixture of CH4 at ambient amount fraction in an N2 matrix) and their known isotopic composition. An open-system Rayleigh model is used to quantify the magnitude of isotopic fractionation affecting measured δ13C(CH4) and δ2H(CH4), which can be used to calculate the possible magnitude of isotopic fractionation given the recovery percentage. These results provide a quantitative understanding of isotopic fractionation during the sample preparation of CH4 from ambient air. The results also provide valuable insights applicable to other cryogenic preconcentration systems, such as those for measurements that probe the distribution of rarer isotopologues.
中文翻译:

环境空气分析制备过程中甲烷同位素体的分馏
从空气中预浓缩甲烷 (CH 4 ) 是测量单取代和双取代同位素体比率的关键采样步骤。我们展示了在常见捕获材料 HayeSep-D 上预浓缩和洗脱过程中同位素分馏的潜力,并研究了其在激光光谱测量中的重要性。通过改变吸附的捕集温度、CH 4通过捕集器的流动方向以及解吸温度斜坡期间 CH 4洗脱的时间,我们解释了影响 δ 13 C(CH 4 ) 和 δ 2 H的分馏背后的机制(CH 4)。结果强调,碳同位素分馏是由平流和扩散驱动的,而氢同位素分馏是由 CH 4与吸附材料的相互作用驱动的(在较高温度下同位素效应趋于较小)。我们比较了样品气体(压缩的全部空气和 N 2基质中环境含量分数的 CH 4合成混合物)的测量同位素比与其已知同位素组成之间的差异。开放系统瑞利模型用于量化影响测量的 δ 13 C(CH 4 ) 和 δ 2 H(CH 4 ) 的同位素分馏的大小,这可用于在给定回收率的情况下计算同位素分馏的可能大小。这些结果提供了对从环境空气中制备 CH 4样品过程中同位素分馏的定量理解。研究结果还提供了适用于其他低温预浓缩系统的宝贵见解,例如用于探测稀有同位素体分布的测量系统。
更新日期:2024-03-22
中文翻译:

环境空气分析制备过程中甲烷同位素体的分馏
从空气中预浓缩甲烷 (CH 4 ) 是测量单取代和双取代同位素体比率的关键采样步骤。我们展示了在常见捕获材料 HayeSep-D 上预浓缩和洗脱过程中同位素分馏的潜力,并研究了其在激光光谱测量中的重要性。通过改变吸附的捕集温度、CH 4通过捕集器的流动方向以及解吸温度斜坡期间 CH 4洗脱的时间,我们解释了影响 δ 13 C(CH 4 ) 和 δ 2 H的分馏背后的机制(CH 4)。结果强调,碳同位素分馏是由平流和扩散驱动的,而氢同位素分馏是由 CH 4与吸附材料的相互作用驱动的(在较高温度下同位素效应趋于较小)。我们比较了样品气体(压缩的全部空气和 N 2基质中环境含量分数的 CH 4合成混合物)的测量同位素比与其已知同位素组成之间的差异。开放系统瑞利模型用于量化影响测量的 δ 13 C(CH 4 ) 和 δ 2 H(CH 4 ) 的同位素分馏的大小,这可用于在给定回收率的情况下计算同位素分馏的可能大小。这些结果提供了对从环境空气中制备 CH 4样品过程中同位素分馏的定量理解。研究结果还提供了适用于其他低温预浓缩系统的宝贵见解,例如用于探测稀有同位素体分布的测量系统。



























 京公网安备 11010802027423号
京公网安备 11010802027423号