当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalytic hydrogenation of acetophenone over Pd–TiO2 using saccharides as hydrogen sources
Catalysis Science & Technology ( IF 5 ) Pub Date : 2024-03-22 , DOI: 10.1039/d4cy00120f Takahiro Oto 1 , Kazuma Ikeuchi 2 , Kosuke Tanaka 1 , Ayumu Onda 3, 4 , Kazuya Imamura 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2024-03-22 , DOI: 10.1039/d4cy00120f Takahiro Oto 1 , Kazuma Ikeuchi 2 , Kosuke Tanaka 1 , Ayumu Onda 3, 4 , Kazuya Imamura 3, 4
Affiliation
|
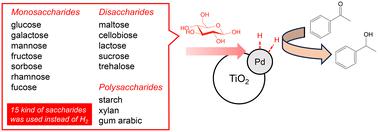
Acetophenone was effectively converted to 1-phenylethanol, a hydrogenated product of acetophenone, over a Pd–TiO2 photocatalyst using various saccharides without the use of hydrogen gas, indicating that various saccharides can be used instead of hydrogen gas in this photocatalytic hydrogenation system. Although 1-phenylethanol was formed when any saccharide was used, the use of saccharides having a hemiacetal structure such as sucrose decreased the reaction rate. This is because that reactivity of the aldehyde (formyl) group is higher in a photocatalytic system using TiO2. Generally, the rate of the reaction is decreased when saccharides with large molecular weight, including oligosaccharides and polysaccharides, are used as substrates in the photocatalytic reaction. However, the rate of the reaction did not depend on the molecular weight of saccharides in the present photocatalytic system. Therefore, this photocatalytic system shows wide applicability of saccharides; 15 kinds of saccharides including 3 kinds of polysaccharides could be used as hydrogen sources. The results indicate the possibility of utilizing biomass for hydrogenation instead of hydrogen gas.
中文翻译:

以糖类为氢源在 Pd-TiO2 上光催化氢化苯乙酮
在Pd-TiO 2光催化剂上,使用各种糖类而不使用氢气,苯乙酮可以有效地转化为苯乙酮的氢化产物1-苯基乙醇,这表明在该光催化氢化系统中可以使用各种糖类代替氢气。尽管当使用任何糖类时都会形成1-苯基乙醇,但是使用具有半缩醛结构的糖类如蔗糖降低了反应速率。这是因为在使用TiO 2的光催化系统中醛(甲酰基)基团的反应性较高。通常,当使用大分子量的糖类(包括寡糖和多糖)作为光催化反应的底物时,反应速率会降低。然而,反应速率并不取决于本光催化体系中糖的分子量。因此,该光催化体系对糖类表现出广泛的适用性;包括3种多糖在内的15种糖类可用作氢源。结果表明利用生物质代替氢气进行加氢的可能性。
更新日期:2024-03-23
中文翻译:

以糖类为氢源在 Pd-TiO2 上光催化氢化苯乙酮
在Pd-TiO 2光催化剂上,使用各种糖类而不使用氢气,苯乙酮可以有效地转化为苯乙酮的氢化产物1-苯基乙醇,这表明在该光催化氢化系统中可以使用各种糖类代替氢气。尽管当使用任何糖类时都会形成1-苯基乙醇,但是使用具有半缩醛结构的糖类如蔗糖降低了反应速率。这是因为在使用TiO 2的光催化系统中醛(甲酰基)基团的反应性较高。通常,当使用大分子量的糖类(包括寡糖和多糖)作为光催化反应的底物时,反应速率会降低。然而,反应速率并不取决于本光催化体系中糖的分子量。因此,该光催化体系对糖类表现出广泛的适用性;包括3种多糖在内的15种糖类可用作氢源。结果表明利用生物质代替氢气进行加氢的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号