Cancer Cell ( IF 50.3 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.ccell.2024.02.011 Qing Deng , Priya Lakra , Panhong Gou , Haopeng Yang , Cem Meydan , Matthew Teater , Christopher Chin , Wenchao Zhang , Tommy Dinh , Usama Hussein , Xubin Li , Estela Rojas , Weiguang Liu , Patrick K. Reville , Atish Kizhakeyil , Darko Barisic , Sydney Parsons , Ashley Wilson , Jared Henderson , Brooks Scull , Channabasavaiah Gurumurthy , Francisco Vega , Amy Chadburn , Branko Cuglievan , Nader Kim El-Mallawany , Carl Allen , Christopher Mason , Ari Melnick , Michael R. Green
|
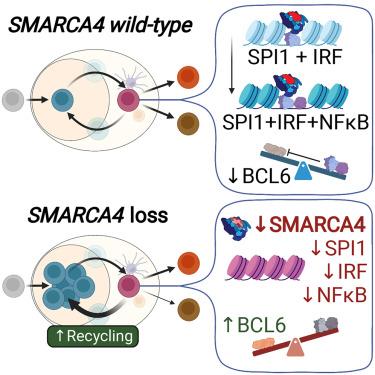
SMARCA4 encodes one of two mutually exclusive ATPase subunits in the BRG/BRM associated factor (BAF) complex that is recruited by transcription factors (TFs) to drive chromatin accessibility and transcriptional activation. SMARCA4 is among the most recurrently mutated genes in human cancer, including ∼30% of germinal center (GC)-derived Burkitt lymphomas. In mice, GC-specific Smarca4 haploinsufficiency cooperated with MYC over-expression to drive lymphomagenesis. Furthermore, monoallelic Smarca4 deletion drove GC hyperplasia with centroblast polarization via significantly increased rates of centrocyte recycling to the dark zone. Mechanistically, Smarca4 loss reduced the activity of TFs that are activated in centrocytes to drive GC-exit, including SPI1 (PU.1), IRF family, and NF-κB. Loss of activity for these factors phenocopied aberrant BCL6 activity within murine centrocytes and human Burkitt lymphoma cells. SMARCA4 therefore facilitates chromatin accessibility for TFs that shape centrocyte trajectories, and loss of fine-control of these programs biases toward centroblast cell-fate, GC hyperplasia and lymphoma.
中文翻译:

SMARCA4 是一种单倍体不足的 B 细胞淋巴瘤肿瘤抑制因子,可微调中心细胞的命运决定
SMARCA4编码 BRG/BRM 相关因子 (BAF) 复合物中两个互斥的 ATP 酶亚基之一,该复合物由转录因子 (TF) 募集以驱动染色质可及性和转录激活。SMARCA4是人类癌症中最常发生突变的基因之一,包括约 30% 的生发中心 (GC) 衍生的伯基特淋巴瘤。在小鼠中,GC 特异性 S marca4单倍体不足与 MYC 过度表达共同驱动淋巴瘤发生。此外,单等位基因Smarca4缺失通过显着增加中心细胞再循环至暗区的速率,驱动 GC 增生和中心母细胞极化。从机制上讲,Smarca4丢失降低了中心细胞中激活的 TF 活性以驱动 GC 退出,包括 SPI1 (PU.1)、IRF 家族和 NF-κB。这些因子活性的丧失导致小鼠中心细胞和人伯基特淋巴瘤细胞内 BCL6 活性异常。因此,SMARCA4 促进了形成中心细胞轨迹的 TF 的染色质可及性,而这些程序的精细控制的丧失会导致中心母细胞命运、GC 增生和淋巴瘤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号