当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Imaging Mass Spectrometry of Isotopically Resolved Intact Proteins on a Trapped Ion-Mobility Quadrupole Time-of-Flight Mass Spectrometer
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.analchem.3c05252 Dustin R. Klein 1, 2 , Emilio S. Rivera 1, 2 , Richard M. Caprioli 1, 2, 3, 4, 5 , Jeffrey M. Spraggins 1, 2, 3, 6, 7
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-22 , DOI: 10.1021/acs.analchem.3c05252 Dustin R. Klein 1, 2 , Emilio S. Rivera 1, 2 , Richard M. Caprioli 1, 2, 3, 4, 5 , Jeffrey M. Spraggins 1, 2, 3, 6, 7
Affiliation
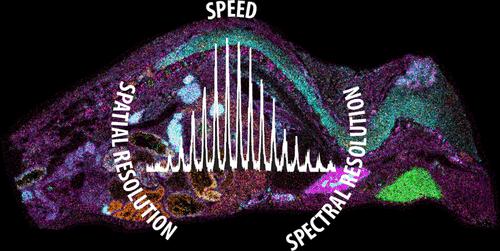
|
In this work, we demonstrate rapid, high spatial, and high spectral resolution imaging of intact proteins by matrix-assisted laser desorption/ionization (MALDI) imaging mass spectrometry (IMS) on a hybrid quadrupole-reflectron time-of-flight (qTOF) mass spectrometer equipped with trapped ion mobility spectrometry (TIMS). Historically, untargeted MALDI IMS of proteins has been performed on TOF mass spectrometers. While advances in TOF instrumentation have enabled rapid, high spatial resolution IMS of intact proteins, TOF mass spectrometers generate relatively low-resolution mass spectra with limited mass accuracy. Conversely, the implementation of MALDI sources on high-resolving power Fourier transform (FT) mass spectrometers has allowed IMS experiments to be conducted with high spectral resolution with the caveat of increasingly long data acquisition times. As illustrated here, qTOF mass spectrometers enable protein imaging with the combined advantages of TOF and FT mass spectrometers. Protein isotope distributions were resolved for both a protein standard mixture and proteins detected from a whole-body mouse pup tissue section. Rapid (∼10 pixels/s) 10 μm lateral spatial resolution IMS was performed on a rat brain tissue section while maintaining isotopic spectral resolution. Lastly, proof-of-concept MALDI-TIMS data was acquired from a protein mixture to demonstrate the ability to differentiate charge states by ion mobility. These experiments highlight the advantages of qTOF and timsTOF platforms for resolving and interpreting complex protein spectra generated from tissue by IMS.
中文翻译:

在捕获离子淌度四极杆飞行时间质谱仪上对同位素解析的完整蛋白质进行成像质谱分析
在这项工作中,我们展示了通过混合四极反射飞行时间(qTOF)上的基质辅助激光解吸/电离(MALDI)成像质谱(IMS)对完整蛋白质进行快速、高空间和高光谱分辨率成像配备捕获离子迁移谱 (TIMS) 的质谱仪。历史上,蛋白质的非靶向 MALDI IMS 是在 TOF 质谱仪上进行的。虽然 TOF 仪器的进步已经实现了完整蛋白质的快速、高空间分辨率 IMS,但 TOF 质谱仪生成的质谱仪分辨率相对较低,质量精度有限。相反,在高分辨率傅里叶变换 (FT) 质谱仪上实施 MALDI 源使得 IMS 实验能够以高光谱分辨率进行,但需要注意的是数据采集时间越来越长。如图所示,qTOF 质谱仪结合了 TOF 和 FT 质谱仪的优点,可实现蛋白质成像。解析了蛋白质标准混合物和从小鼠全身组织切片中检测到的蛋白质的蛋白质同位素分布。在大鼠脑组织切片上进行快速(~10 像素/秒)10 μm 横向空间分辨率 IMS,同时保持同位素光谱分辨率。最后,从蛋白质混合物中获取概念验证 MALDI-TIMS 数据,以证明通过离子淌度区分电荷状态的能力。这些实验凸显了 qTOF 和 timsTOF 平台在解析和解释 IMS 从组织生成的复杂蛋白质光谱方面的优势。
更新日期:2024-03-22
中文翻译:

在捕获离子淌度四极杆飞行时间质谱仪上对同位素解析的完整蛋白质进行成像质谱分析
在这项工作中,我们展示了通过混合四极反射飞行时间(qTOF)上的基质辅助激光解吸/电离(MALDI)成像质谱(IMS)对完整蛋白质进行快速、高空间和高光谱分辨率成像配备捕获离子迁移谱 (TIMS) 的质谱仪。历史上,蛋白质的非靶向 MALDI IMS 是在 TOF 质谱仪上进行的。虽然 TOF 仪器的进步已经实现了完整蛋白质的快速、高空间分辨率 IMS,但 TOF 质谱仪生成的质谱仪分辨率相对较低,质量精度有限。相反,在高分辨率傅里叶变换 (FT) 质谱仪上实施 MALDI 源使得 IMS 实验能够以高光谱分辨率进行,但需要注意的是数据采集时间越来越长。如图所示,qTOF 质谱仪结合了 TOF 和 FT 质谱仪的优点,可实现蛋白质成像。解析了蛋白质标准混合物和从小鼠全身组织切片中检测到的蛋白质的蛋白质同位素分布。在大鼠脑组织切片上进行快速(~10 像素/秒)10 μm 横向空间分辨率 IMS,同时保持同位素光谱分辨率。最后,从蛋白质混合物中获取概念验证 MALDI-TIMS 数据,以证明通过离子淌度区分电荷状态的能力。这些实验凸显了 qTOF 和 timsTOF 平台在解析和解释 IMS 从组织生成的复杂蛋白质光谱方面的优势。



























 京公网安备 11010802027423号
京公网安备 11010802027423号