当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Vitro and In Silico Explorations of the Protein Conformational Changes of Corynebacterium glutamicum MshA, a Model Retaining GT-B Glycosyltransferase
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.biochem.3c00561 Bakar A. Hassan 1 , Jozafina Milicaj 1 , Meka Tyson 1 , Ramesh Karki 2 , Yuk Y. Sham 3, 4 , Patrick A. Frantom 2 , Erika A. Taylor 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.biochem.3c00561 Bakar A. Hassan 1 , Jozafina Milicaj 1 , Meka Tyson 1 , Ramesh Karki 2 , Yuk Y. Sham 3, 4 , Patrick A. Frantom 2 , Erika A. Taylor 1
Affiliation
|
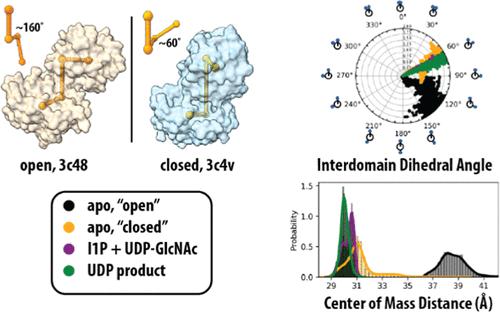
MshA is a GT-B glycosyltransferase catalyzing the first step in the biosynthesis of mycothiol. While many GT-B enzymes undergo an open-to-closed transition, MshA is unique because its 97° rotation is beyond the usual range of 10–25°. Molecular dynamics (MD) simulations were carried out for MshA in both ligand bound and unbound states to investigate the effect of ligand binding on localized protein dynamics and its conformational free energy landscape. Simulations showed that both the unliganded “opened” and liganded “closed” forms of the enzyme sample a wide degree of dihedral angles and interdomain distances with relatively low overlapping populations. Calculation of the free energy surface using replica exchange MD for the apo “opened” and an artificial generated apo “closed” structure revealed overlaps in the geometries sampled, allowing calculation of a barrier of 2 kcal/mol for the open-to-closed transition in the absence of ligands. MD simulations of fully liganded MshA revealed a smaller sampling of the dihedral angles. The localized protein fluctuation changes suggest that UDP-GlcNAc binding activates the motions of loops in the 1-l-myo-inositol-1-phosphate (I1P)-binding site despite little change in the interactions with UDP-GlcNAc. Circular dichroism, intrinsic fluorescence spectroscopy, and mutagenesis studies were used to confirm the ligand-induced structural changes in MshA. The results support a proposed mechanism where UDP-GlcNAc binds with rigid interactions to the C-terminal domain of MshA and activates flexible loops in the N-terminal domain for binding and positioning of I1P. This model can be used for future structure-based drug development of inhibitors of the mycothiol biosynthetic pathway.
中文翻译:

保留 GT-B 糖基转移酶的模型谷氨酸棒杆菌 MshA 蛋白质构象变化的体外和计算机探索
MshA 是一种 GT-B 糖基转移酶,催化菌硫醇生物合成的第一步。虽然许多 GT-B 酶经历开放到封闭的转变,但 MshA 是独一无二的,因为它的 97° 旋转超出了通常的 10-25° 范围。对配体结合和未结合状态的 MshA 进行分子动力学 (MD) 模拟,以研究配体结合对局部蛋白质动力学及其构象自由能景观的影响。模拟表明,未配体的“开放”形式和配体的“闭合”形式的酶样品都具有较宽的二面角和域间距离,并且重叠群体相对较低。使用apo“打开”的复制品交换MD和人工生成的apo“闭合”结构计算自由能表面,揭示了采样几何形状中的重叠,允许计算打开到闭合转变的2 kcal/mol的势垒在没有配体的情况下。完全配体的 MshA 的 MD 模拟揭示了二面角的较小样本。局部蛋白质波动变化表明,尽管与 UDP-GlcNAc 的相互作用几乎没有变化,但 UDP-GlcNAc 结合激活了 1 - l-肌醇-1-磷酸 (I1P) 结合位点中环的运动。使用圆二色性、内在荧光光谱和诱变研究来确认配体诱导的 MshA 结构变化。结果支持了一种提出的机制,其中 UDP-GlcNAc 以刚性相互作用结合到 MshA 的 C 端结构域,并激活 N 端结构域中的柔性环以结合和定位 I1P。该模型可用于未来基于结构的菌硫醇生物合成途径抑制剂的药物开发。
更新日期:2024-03-20
中文翻译:

保留 GT-B 糖基转移酶的模型谷氨酸棒杆菌 MshA 蛋白质构象变化的体外和计算机探索
MshA 是一种 GT-B 糖基转移酶,催化菌硫醇生物合成的第一步。虽然许多 GT-B 酶经历开放到封闭的转变,但 MshA 是独一无二的,因为它的 97° 旋转超出了通常的 10-25° 范围。对配体结合和未结合状态的 MshA 进行分子动力学 (MD) 模拟,以研究配体结合对局部蛋白质动力学及其构象自由能景观的影响。模拟表明,未配体的“开放”形式和配体的“闭合”形式的酶样品都具有较宽的二面角和域间距离,并且重叠群体相对较低。使用apo“打开”的复制品交换MD和人工生成的apo“闭合”结构计算自由能表面,揭示了采样几何形状中的重叠,允许计算打开到闭合转变的2 kcal/mol的势垒在没有配体的情况下。完全配体的 MshA 的 MD 模拟揭示了二面角的较小样本。局部蛋白质波动变化表明,尽管与 UDP-GlcNAc 的相互作用几乎没有变化,但 UDP-GlcNAc 结合激活了 1 - l-肌醇-1-磷酸 (I1P) 结合位点中环的运动。使用圆二色性、内在荧光光谱和诱变研究来确认配体诱导的 MshA 结构变化。结果支持了一种提出的机制,其中 UDP-GlcNAc 以刚性相互作用结合到 MshA 的 C 端结构域,并激活 N 端结构域中的柔性环以结合和定位 I1P。该模型可用于未来基于结构的菌硫醇生物合成途径抑制剂的药物开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号