当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cationic [1,5]-Aryl Migrations of Propargyl Benzyl Ethers – A Stereospecific Approach to E- and Z-Tetrasubstituted Olefins
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-22 , DOI: 10.1002/adsc.202400030 Beeraiah Baire 1 , Sindoori R Nair 1 , Santu Sadhukhan 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-22 , DOI: 10.1002/adsc.202400030 Beeraiah Baire 1 , Sindoori R Nair 1 , Santu Sadhukhan 2
Affiliation
|
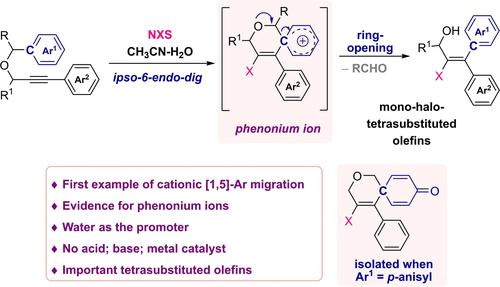
. Herein we report the discovery and development of the first example of a cationic [1,5]‐aryl migration reaction, through an unprecedented phenonium ions. This reaction does not require any acid, base, transition metal‐based catalyst or promoter, instead promoted by the water activated NXS reagents. It provides a highly diastereoselective and rapid access to the medicinally and biologically important tetra‐substituted olefins (allyl alcohols). The involvement of an ipso‐carbon, as well as the phenonium ion during this novel, cationic [1,5]‐aryl migration was strongly supported by the isolation of 2‐oxaspiro[5.5]undecane‐9‐ones. This methodology is highly tolerable for a wide range of propargyl‐benzyl ethers, resulting in a selective construction of structurally divergent library of mono‐halo‐tricarbon‐substituted alkenes. Synthetic application has also been demonstrated by converting two isomeric‐iodo‐olefins to respective E‐ and Z‐isomers of tamoxifen in a stereospecific manner.
中文翻译:

炔丙基苄基醚的阳离子[1,5]-芳基迁移——E-和Z-四取代烯烃的立体定向方法
。在此,我们报告了通过前所未有的苯鎓离子进行阳离子[1,5]-芳基迁移反应的第一个例子的发现和发展。该反应不需要任何酸、碱、过渡金属催化剂或促进剂,而是由水活化的 NXS 试剂促进。它提供了高度非对映选择性并快速获得具有医学和生物学意义的四取代烯烃(烯丙醇)。 2-氧杂螺[5.5]十一烷-9-酮的分离有力地支持了在这种新颖的阳离子[1,5]-芳基迁移过程中,异碳以及苯鎓离子的参与。该方法对于各种炔丙基苄基醚具有高度的耐受性,从而选择性地构建结构不同的单卤代三碳取代烯烃库。通过以立体定向方式将两种异构碘代烯烃转化为他莫昔芬各自的 E 和 Z 异构体,也证明了合成应用。
更新日期:2024-03-22
中文翻译:

炔丙基苄基醚的阳离子[1,5]-芳基迁移——E-和Z-四取代烯烃的立体定向方法
。在此,我们报告了通过前所未有的苯鎓离子进行阳离子[1,5]-芳基迁移反应的第一个例子的发现和发展。该反应不需要任何酸、碱、过渡金属催化剂或促进剂,而是由水活化的 NXS 试剂促进。它提供了高度非对映选择性并快速获得具有医学和生物学意义的四取代烯烃(烯丙醇)。 2-氧杂螺[5.5]十一烷-9-酮的分离有力地支持了在这种新颖的阳离子[1,5]-芳基迁移过程中,异碳以及苯鎓离子的参与。该方法对于各种炔丙基苄基醚具有高度的耐受性,从而选择性地构建结构不同的单卤代三碳取代烯烃库。通过以立体定向方式将两种异构碘代烯烃转化为他莫昔芬各自的 E 和 Z 异构体,也证明了合成应用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号