当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aziridination via Nitrogen-Atom Transfer to Olefins from Photoexcited Azoxy-Triazenes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-24 , DOI: 10.1021/jacs.3c14713 Joshua K. Mitchell 1 , Waseem A. Hussain 1 , Ajay H. Bansode 1 , Ryan M. O’Connor 1 , Marvin Parasram 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-24 , DOI: 10.1021/jacs.3c14713 Joshua K. Mitchell 1 , Waseem A. Hussain 1 , Ajay H. Bansode 1 , Ryan M. O’Connor 1 , Marvin Parasram 1
Affiliation
|
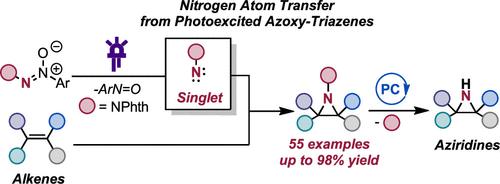
Herein, we report that readily accessible azoxy-triazenes can serve as nitrogen atom sources under visible light excitation for the phthalimido-protected aziridination of alkenes. This approach eliminates the need for external oxidants, precious transition metals, and photocatalysts, marking a departure from conventional methods. The versatility of this transformation extends to the selective aziridination of both activated and unactivated multisubstituted alkenes of varying electronic profiles. Notably, this process avoids the formation of competing C–H insertion products. The described protocol is operationally simple, scalable, and adaptable to photoflow conditions. Mechanistic studies support the idea that the photofragmentation of azoxy-triazenes results in the generation of a free singlet nitrene. Furthermore, a mild photoredox-catalyzed N–N cleavage of the protecting group to furnish the free aziridines is reported. Our findings contribute to the advancement of sustainable and practical methodologies for the synthesis of nitrogen-containing compounds, showcasing the potential for broader applications in synthetic chemistry.
中文翻译:

通过氮原子从光激发偶氮三氮烯转移至烯烃的氮丙啶化
在此,我们报道了容易获得的氧化偶氮三氮烯可以在可见光激发下作为氮原子源,用于邻苯二甲酰亚胺基保护的烯烃的氮丙啶化。这种方法不需要外部氧化剂、贵重过渡金属和光催化剂,这标志着与传统方法的不同。这种转化的多功能性延伸到不同电子谱的活化和未活化多取代烯烃的选择性氮丙啶化。值得注意的是,这个过程避免了竞争性 C-H 插入产物的形成。所描述的协议操作简单、可扩展且适用于照片流条件。机理研究支持氧化偶氮三氮烯的光裂解导致游离单线态氮烯的产生的观点。此外,据报道,保护基团在光氧化还原催化下发生温和的 N-N 裂解,得到游离的氮丙啶。我们的研究结果有助于推进含氮化合物合成的可持续和实用方法,展示了在合成化学中更广泛应用的潜力。
更新日期:2024-03-24
中文翻译:

通过氮原子从光激发偶氮三氮烯转移至烯烃的氮丙啶化
在此,我们报道了容易获得的氧化偶氮三氮烯可以在可见光激发下作为氮原子源,用于邻苯二甲酰亚胺基保护的烯烃的氮丙啶化。这种方法不需要外部氧化剂、贵重过渡金属和光催化剂,这标志着与传统方法的不同。这种转化的多功能性延伸到不同电子谱的活化和未活化多取代烯烃的选择性氮丙啶化。值得注意的是,这个过程避免了竞争性 C-H 插入产物的形成。所描述的协议操作简单、可扩展且适用于照片流条件。机理研究支持氧化偶氮三氮烯的光裂解导致游离单线态氮烯的产生的观点。此外,据报道,保护基团在光氧化还原催化下发生温和的 N-N 裂解,得到游离的氮丙啶。我们的研究结果有助于推进含氮化合物合成的可持续和实用方法,展示了在合成化学中更广泛应用的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号