当前位置:
X-MOL 学术
›
Int. J. Hydrogen Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and kinetic modeling study on laminar flame speeds and emission characteristics of oxy-ammonia premixed flames
International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2024-03-22 , DOI: 10.1016/j.ijhydene.2024.03.042 Yu Zhang , Wenda Zhang , Boshuai Yu , Xincheng Li , Linyao Zhang , Yijun Zhao , Shaozeng Sun
International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2024-03-22 , DOI: 10.1016/j.ijhydene.2024.03.042 Yu Zhang , Wenda Zhang , Boshuai Yu , Xincheng Li , Linyao Zhang , Yijun Zhao , Shaozeng Sun
|
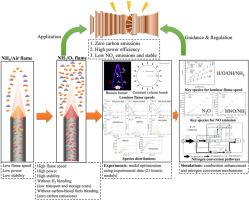
The low flame speed and NO emissions of ammonia are the key to affecting combustion temperature, stability, flame structure, and pollutant control, limiting its application in power generation devices. This study proposes oxy-ammonia combustion (oxygen content in oxidant = 100%, NH/O combustion) as a novel method for significantly enhancing ammonia combustion. A method for measuring the laminar flame speed using the NH* distribution of the Bunsen burner flame is proposed, and the measured data is close to the constant volume combustion bomb. 21 kinetic models were collected and optimized after comparison with the experimental data of constant volume combustion bombs, and results show that Han et al.'s model has higher prediction accuracy for NH/O flames. The preheating temperature was relevant to gas turbine combustors (298.15–500 K), and the equivalence ratio ranged from 0.7 to 1.6. At this extreme condition (oxygen content in oxidant = 100%), the results show that the maximum laminar flame speed reaches 1.25 m/s at ambient pressure and temperature, which is approximately 18 times that of NH/air combustion, mainly due to the increased reaction rates of OH, H, O and NH radicals in the reaction zone (primary ammonia decomposition and H/O radical pool). The NO emission of NH/O combustion at stoichiometric conditions is about 7216 ppmv@15%O, 1.5 and 13 times that of NH/air and CH/air combustion, respectively. NO production and consumption may follow the NO and NNH mechanism, and HNO is an important precursor for NO formation. Increasing the preheating temperature has less effect on the reaction pathway directions in the reaction network of nitrogen conversion but affects the path flux significantly.
中文翻译:

氧氨预混火焰层流火焰速度和发射特性的实验和动力学模拟研究
氨的低火焰速度和NO排放是影响燃烧温度、稳定性、火焰结构和污染物控制的关键,限制了其在发电装置中的应用。本研究提出氧氨燃烧(氧化剂中氧含量 = 100%,NH/O 燃烧)作为显着增强氨燃烧的新方法。提出了利用本生灯火焰NH*分布测量层流火焰速度的方法,测量数据接近定容燃烧弹。收集并优化了21个动力学模型,与定容燃烧弹的实验数据进行对比,结果表明Han等人的模型对NH/O火焰具有较高的预测精度。预热温度与燃气轮机燃烧室相关(298.15~500 K),当量比范围为0.7~1.6。在这种极端条件下(氧化剂中的氧含量=100%),结果表明,在环境压力和温度下,最大层流火焰速度达到1.25 m/s,大约是NH/空气燃烧的18倍,这主要是由于提高反应区(初级氨分解和H/O自由基池)中OH、H、O和NH自由基的反应速率。在化学计量条件下,NH/O燃烧的NO排放量约为7216 ppmv@15%O,分别是NH/空气和CH/空气燃烧的1.5和13倍。 NO的产生和消耗可能遵循NO和NNH机制,而HNO是NO形成的重要前体。提高预热温度对氮转化反应网络中的反应路径方向影响较小,但对路径通量影响较大。
更新日期:2024-03-22
中文翻译:

氧氨预混火焰层流火焰速度和发射特性的实验和动力学模拟研究
氨的低火焰速度和NO排放是影响燃烧温度、稳定性、火焰结构和污染物控制的关键,限制了其在发电装置中的应用。本研究提出氧氨燃烧(氧化剂中氧含量 = 100%,NH/O 燃烧)作为显着增强氨燃烧的新方法。提出了利用本生灯火焰NH*分布测量层流火焰速度的方法,测量数据接近定容燃烧弹。收集并优化了21个动力学模型,与定容燃烧弹的实验数据进行对比,结果表明Han等人的模型对NH/O火焰具有较高的预测精度。预热温度与燃气轮机燃烧室相关(298.15~500 K),当量比范围为0.7~1.6。在这种极端条件下(氧化剂中的氧含量=100%),结果表明,在环境压力和温度下,最大层流火焰速度达到1.25 m/s,大约是NH/空气燃烧的18倍,这主要是由于提高反应区(初级氨分解和H/O自由基池)中OH、H、O和NH自由基的反应速率。在化学计量条件下,NH/O燃烧的NO排放量约为7216 ppmv@15%O,分别是NH/空气和CH/空气燃烧的1.5和13倍。 NO的产生和消耗可能遵循NO和NNH机制,而HNO是NO形成的重要前体。提高预热温度对氮转化反应网络中的反应路径方向影响较小,但对路径通量影响较大。



























 京公网安备 11010802027423号
京公网安备 11010802027423号