当前位置:
X-MOL 学术
›
Int. J. Hydrogen Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
First principles study of hydrogen storage on B-doped SiC monolayers through light transition metal atoms
International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2024-03-21 , DOI: 10.1016/j.ijhydene.2024.03.133 Francisco De Santiago , Lucia G. Arellano , Ivonne J. Hernández-Hernández , Alma R. Heredia , Álvaro Miranda , Alejandro Trejo , Luis A. Pérez , Miguel Cruz-Irisson
International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2024-03-21 , DOI: 10.1016/j.ijhydene.2024.03.133 Francisco De Santiago , Lucia G. Arellano , Ivonne J. Hernández-Hernández , Alma R. Heredia , Álvaro Miranda , Alejandro Trejo , Luis A. Pérez , Miguel Cruz-Irisson
|
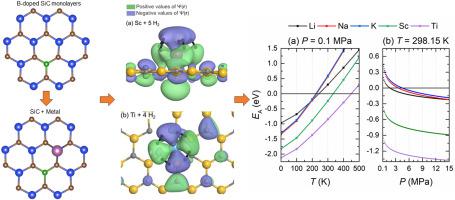
In this first-principles study, based on Density Functional Theory, we assess the capacity of metal-decorated, boron-doped, graphene-like monolayers of silicon carbide (SiC) to adsorb hydrogen molecules. To enhance the binding of metal adatoms on SiC monolayers, these were substitutionally doped with boron atoms. Alkaline, alkaline-earth, and transition metal adatoms were considered and their hydrogen storage capabilities were compared. The results show that alkaline-earth metal adatoms are not suitable for hydrogen storage. On the other hand, sodium- and potassium-decorated B-doped SiC monolayers adsorb the largest number of H molecules per adatom, but their adsorption energies are insufficient for an adequate hydrogen storage. Titanium and scandium adatoms are the most suitable for hydrogen storage since they exhibit good adsorption energies and up to four and five H molecules per adatom, respectively. Moreover, the estimated potential barriers for diffusion of these two adatoms on the B-doped SiC monolayers indicate that the probability of clustering is very low. Moreover, within the ideal-gas approximation, it is estimated that hydrogen can be stored in the Ti- and Sc-decorated monolayers at room temperature and atmospheric pressure. Furthermore, if SiC monolayers were doped with boron atoms in concentrations similar to those reported for graphene, it is estimated that the gravimetric capacities could reach 5.1 wt% and 6.3 wt% for Ti-decorated and Sc-decorated monolayers, respectively, which are close to the target hydrogen-storage capacities envisioned for the near future.
中文翻译:

B掺杂SiC单层轻过渡金属原子储氢的第一性原理研究
在这项基于密度泛函理论的第一性原理研究中,我们评估了金属装饰、硼掺杂、类石墨烯单层碳化硅 (SiC) 吸附氢分子的能力。为了增强金属吸附原子在碳化硅单层上的结合,用硼原子进行替代掺杂。考虑了碱性、碱土和过渡金属吸附原子,并比较了它们的储氢能力。结果表明,碱土金属吸附原子不适合储氢。另一方面,钠和钾修饰的B掺杂SiC单层每个吸附原子吸附最大数量的H分子,但它们的吸附能不足以存储足够的氢。钛和钪吸附原子最适合储氢,因为它们具有良好的吸附能,每个吸附原子分别最多有四个和五个氢分子。此外,估计的这两种吸附原子在 B 掺杂 SiC 单层上扩散的势垒表明,聚类的可能性非常低。此外,在理想气体近似下,估计氢可以在室温和大气压下储存在Ti和Sc装饰的单层中。此外,如果 SiC 单层掺杂硼原子的浓度与石墨烯报道的浓度相似,则估计 Ti 装饰和 Sc 装饰单层的重量容量可分别达到 5.1 wt% 和 6.3 wt%,两者非常接近。达到近期设想的储氢能力目标。
更新日期:2024-03-21
中文翻译:

B掺杂SiC单层轻过渡金属原子储氢的第一性原理研究
在这项基于密度泛函理论的第一性原理研究中,我们评估了金属装饰、硼掺杂、类石墨烯单层碳化硅 (SiC) 吸附氢分子的能力。为了增强金属吸附原子在碳化硅单层上的结合,用硼原子进行替代掺杂。考虑了碱性、碱土和过渡金属吸附原子,并比较了它们的储氢能力。结果表明,碱土金属吸附原子不适合储氢。另一方面,钠和钾修饰的B掺杂SiC单层每个吸附原子吸附最大数量的H分子,但它们的吸附能不足以存储足够的氢。钛和钪吸附原子最适合储氢,因为它们具有良好的吸附能,每个吸附原子分别最多有四个和五个氢分子。此外,估计的这两种吸附原子在 B 掺杂 SiC 单层上扩散的势垒表明,聚类的可能性非常低。此外,在理想气体近似下,估计氢可以在室温和大气压下储存在Ti和Sc装饰的单层中。此外,如果 SiC 单层掺杂硼原子的浓度与石墨烯报道的浓度相似,则估计 Ti 装饰和 Sc 装饰单层的重量容量可分别达到 5.1 wt% 和 6.3 wt%,两者非常接近。达到近期设想的储氢能力目标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号