当前位置:
X-MOL 学术
›
Int. J. Hydrogen Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of cation in catalytic decomposition of ammonia over Ni supported zeolite Y catalysts
International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2024-03-21 , DOI: 10.1016/j.ijhydene.2024.03.204 Shaofeng Gong , Zexue Du , Yi Hu , Wenwu Yao
International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2024-03-21 , DOI: 10.1016/j.ijhydene.2024.03.204 Shaofeng Gong , Zexue Du , Yi Hu , Wenwu Yao
|
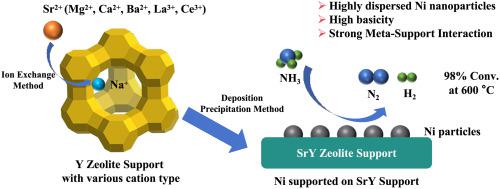
A series of nickel (Ni) catalysts were prepared using zeolites Y with different cation types (Na, Mg, Ca, Sr, Ba, Ce, and La) as supports and characterized by using XRD, BET, ICP-OES, XPS, and , as well as NH-TPD, CO-TPD, and H-TPR techniques. Their catalytic performances in decomposition of ammonia (NH) to hydrogen (H) were evaluated with a fixed-bed flow reactor system. The catalytic activities of the prepared catalysts for NH decomposition decreased in the order of Ni/SrY > Ni/BaY > Ni/LaY > Ni/CaY > Ni/NaY > Ni/MgY > Ni/CeY. The high activity of Ni/SrY catalyst may be attributed to its small Ni nanoparticle size, high basicity and strong interaction between highly dispersed Ni nanoparticles and SrY support. At 600 °C and GHSV of 9000 mLNH·g·h, 98.0 % NH conversion with about 105.2 mmol min·g H formation rate can be accomplished over Ni/SrY catalyst.
中文翻译:

阳离子在 Ni 负载沸石 Y 催化剂上催化分解氨中的作用
以不同阳离子类型(Na、Mg、Ca、Sr、Ba、Ce、La)的 Y 型沸石为载体制备了一系列镍(Ni)催化剂,并通过 XRD、BET、ICP-OES、XPS 和,以及 NH-TPD、CO-TPD 和 H-TPR 技术。使用固定床流动反应器系统评估了它们在氨(NH)分解为氢气(H)方面的催化性能。所制备的催化剂对NH分解的催化活性依次降低为Ni/SrY > Ni/BaY > Ni/LaY > Ni/CaY > Ni/NaY > Ni/MgY > Ni/CeY。 Ni/SrY催化剂的高活性可能归因于其较小的Ni纳米颗粒尺寸、较高的碱度以及高度分散的Ni纳米颗粒与SrY载体之间的强相互作用。在 600 °C 和 9000 mLNH·g·h 的 GHSV 下,通过 Ni/SrY 催化剂可以实现 98.0 % NH 转化率和约 105.2 mmol min·g H 生成速率。
更新日期:2024-03-21
中文翻译:

阳离子在 Ni 负载沸石 Y 催化剂上催化分解氨中的作用
以不同阳离子类型(Na、Mg、Ca、Sr、Ba、Ce、La)的 Y 型沸石为载体制备了一系列镍(Ni)催化剂,并通过 XRD、BET、ICP-OES、XPS 和,以及 NH-TPD、CO-TPD 和 H-TPR 技术。使用固定床流动反应器系统评估了它们在氨(NH)分解为氢气(H)方面的催化性能。所制备的催化剂对NH分解的催化活性依次降低为Ni/SrY > Ni/BaY > Ni/LaY > Ni/CaY > Ni/NaY > Ni/MgY > Ni/CeY。 Ni/SrY催化剂的高活性可能归因于其较小的Ni纳米颗粒尺寸、较高的碱度以及高度分散的Ni纳米颗粒与SrY载体之间的强相互作用。在 600 °C 和 9000 mLNH·g·h 的 GHSV 下,通过 Ni/SrY 催化剂可以实现 98.0 % NH 转化率和约 105.2 mmol min·g H 生成速率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号