当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Breaking Sabatier's vertex via switching the oxygen adsorption configuration and reaction pathway on dual active sites for acidic oxygen reduction
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-03-25 , DOI: 10.1039/d4ee00823e Pan Guo 1 , Bo Liu 1 , Fengdi Tu 1 , Yunkun Dai 1 , Ziyu Zhang 1 , Yunfei Xia 1 , Miao Ma 1 , Yunlong Zhang 1 , Lei Zhao 1 , Zhenbo Wang 1
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-03-25 , DOI: 10.1039/d4ee00823e Pan Guo 1 , Bo Liu 1 , Fengdi Tu 1 , Yunkun Dai 1 , Ziyu Zhang 1 , Yunfei Xia 1 , Miao Ma 1 , Yunlong Zhang 1 , Lei Zhao 1 , Zhenbo Wang 1
Affiliation
|
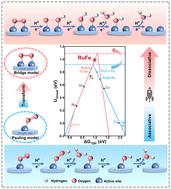
Single-atom catalysts are promising alternatives to platinum-based catalysts for the oxygen reduction reaction (ORR). However, the ORR process with multiple-step proton-coupled electron transfer occurring on a single-active site follows the linear scaling relation, making it difficult to break through Sabatier's limitation. Herein, we switch the ORR process from a sluggish associative pathway to a favorable dissociative one by constructing diatomic active sites with a Pt-like adsorption configuration, enabling the thermodynamic limit potential to break through Sabatier's vertex. Theoretical calculations and in situ characterization fully corroborate the Pt-like adsorption configuration of O2 on Ru–Fe dual sites, which renders the direct cleavage of O–O bonds and avoids the formation of *OOH intermediates, thus boosting the ORR kinetics. Consequently, the well-designed Ru and Fe co-doped catalysts with dual active sites (Ru, Fe-NC DAS) deliver extraordinary ORR catalytic performance, as manifested by the high half-wave potential of 0.843 V in an acid medium and a record-breaking peak power density of 1.152 W cm−2 in H2/O2 fuel cells, ranking at the top level of non-Pt catalysts reported so far. This work provides a new approach for designing highly efficient atomically dispersed catalysts and steering the corresponding catalytic reaction mechanisms.
中文翻译:

通过切换酸性氧还原双活性位点上的氧吸附构型和反应路径来打破萨巴蒂尔顶点
单原子催化剂是用于氧还原反应(ORR)的铂基催化剂的有前途的替代品。然而,在单个活性位点上发生的多步质子耦合电子转移的ORR过程遵循线性标度关系,因此很难突破Sabatier的限制。在此,我们通过构建具有类 Pt 吸附构型的双原子活性位点,将 ORR 过程从缓慢的缔合途径转变为有利的解离途径,从而使热力学极限势能突破 Sabatier 顶点。理论计算和原位表征充分证实了O 2在Ru-Fe双位点上的类Pt吸附构型,使得O-O键直接断裂,避免了*OOH中间体的形成,从而提高了ORR动力学。因此,精心设计的具有双活性位点的 Ru 和 Fe 共掺杂催化剂(Ru、Fe-NC DAS)具有非凡的 ORR 催化性能,在酸性介质中半波电位高达 0.843 V,创下了记录。 -在H 2 /O 2燃料电池中突破峰值功率密度1.152 W cm -2,位居迄今为止报道的非Pt催化剂的最高水平。这项工作为设计高效原子分散催化剂和控制相应的催化反应机制提供了一种新方法。
更新日期:2024-03-25
中文翻译:

通过切换酸性氧还原双活性位点上的氧吸附构型和反应路径来打破萨巴蒂尔顶点
单原子催化剂是用于氧还原反应(ORR)的铂基催化剂的有前途的替代品。然而,在单个活性位点上发生的多步质子耦合电子转移的ORR过程遵循线性标度关系,因此很难突破Sabatier的限制。在此,我们通过构建具有类 Pt 吸附构型的双原子活性位点,将 ORR 过程从缓慢的缔合途径转变为有利的解离途径,从而使热力学极限势能突破 Sabatier 顶点。理论计算和原位表征充分证实了O 2在Ru-Fe双位点上的类Pt吸附构型,使得O-O键直接断裂,避免了*OOH中间体的形成,从而提高了ORR动力学。因此,精心设计的具有双活性位点的 Ru 和 Fe 共掺杂催化剂(Ru、Fe-NC DAS)具有非凡的 ORR 催化性能,在酸性介质中半波电位高达 0.843 V,创下了记录。 -在H 2 /O 2燃料电池中突破峰值功率密度1.152 W cm -2,位居迄今为止报道的非Pt催化剂的最高水平。这项工作为设计高效原子分散催化剂和控制相应的催化反应机制提供了一种新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号