当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism-Guided Discovery of Cleavable Comonomers for Backbone Deconstructable Poly(methyl methacrylate)
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-25 , DOI: 10.1021/jacs.3c14554 Kwangwook Ko 1 , David J. Lundberg 2 , Alayna M. Johnson 1 , Jeremiah A. Johnson 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-25 , DOI: 10.1021/jacs.3c14554 Kwangwook Ko 1 , David J. Lundberg 2 , Alayna M. Johnson 1 , Jeremiah A. Johnson 1
Affiliation
|
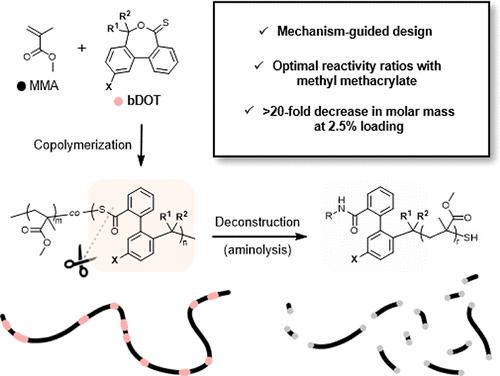
The development of cleavable comonomers (CCs) with suitable copolymerization reactivity paves the way for the introduction of backbone deconstructability into polymers. Recent advancements in thionolactone-based CCs, exemplified by dibenzo[c,e]-oxepine-5(7H)-thione (DOT), have opened promising avenues for the selective deconstruction of multiple classes of vinyl polymers, including polyacrylates, polyacrylamides, and polystyrenics. To date, however, no thionolactone CC has been shown to copolymerize with methacrylates to an appreciable extent to enable polymer deconstruction. Here, we overcome this challenge through the design of a new class of benzyl-functionalized thionolactones (bDOTs). Guided by detailed mechanistic analyses, we find that the introduction of radical-stabilizing substituents to bDOTs enables markedly increased and tunable copolymerization reactivity with methyl methacrylate (MMA). Through iterative optimizations of the molecular structure, a specific bDOT, F-p-CF3PhDOT, is discovered to copolymerize efficiently with MMA. High molar mass deconstructable PMMA-based copolymers (dPMMA, Mn > 120 kDa) with low percentages of F-p-CF3PhDOT (1.8 and 3.8 mol%) are prepared using industrially relevant bulk free radical copolymerization conditions. The thermomechanical properties of dPMMA are similar to PMMA; however, the former is shown to degrade into low molar mass fragments (<6.5 kDa) under mild aminolysis conditions. This work presents the first example of a radical ring-opening CC capable of nearly random copolymerization with MMA without the possibility of cross-linking and provides a workflow for the mechanism-guided design of deconstructable copolymers in the future.
中文翻译:

主链可解构聚(甲基丙烯酸甲酯)可裂解共聚单体的机制引导发现
具有合适共聚反应性的可裂解共聚单体(CC)的开发为将主链可解构性引入聚合物铺平了道路。以二苯并[c,e]-氧杂环己烷-5(7H)-硫酮 (DOT) 为代表的基于硫醇内酯的 CC 的最新进展,为选择性解构多类乙烯基聚合物(包括聚丙烯酸酯、聚丙烯酰胺和聚苯乙烯。然而,迄今为止,尚未发现硫醇内酯 CC 能够与甲基丙烯酸酯共聚到可观的程度以实现聚合物解构。在这里,我们通过设计一类新型的苄基官能化硫醇内酯(bDOT)来克服这一挑战。在详细的机理分析的指导下,我们发现在 bDOT 中引入自由基稳定取代基可以显着提高与甲基丙烯酸甲酯 (MMA) 的可调节共聚反应性。通过分子结构的迭代优化,发现一种特定的bDOT, F - p -CF 3 PhDOT ,可以与MMA有效共聚。使用工业相关的本体自由基共聚条件制备具有低百分比F- p -CF 3 Ph 3 PhDOT (1.8 和 3.8 mol%)的高摩尔质量可解构 PMMA 基共聚物(dPMMA,M n > 120 kDa)。 dPMMA的热机械性能与PMMA相似;然而,前者在温和的氨解条件下会降解成低摩尔质量片段(<6.5 kDa)。这项工作提出了第一个自由基开环 CC 能够与 MMA 几乎无规共聚而无需交联的例子,并为未来可解构共聚物的机制引导设计提供了工作流程。
更新日期:2024-03-25
中文翻译:

主链可解构聚(甲基丙烯酸甲酯)可裂解共聚单体的机制引导发现
具有合适共聚反应性的可裂解共聚单体(CC)的开发为将主链可解构性引入聚合物铺平了道路。以二苯并[c,e]-氧杂环己烷-5(7H)-硫酮 (DOT) 为代表的基于硫醇内酯的 CC 的最新进展,为选择性解构多类乙烯基聚合物(包括聚丙烯酸酯、聚丙烯酰胺和聚苯乙烯。然而,迄今为止,尚未发现硫醇内酯 CC 能够与甲基丙烯酸酯共聚到可观的程度以实现聚合物解构。在这里,我们通过设计一类新型的苄基官能化硫醇内酯(bDOT)来克服这一挑战。在详细的机理分析的指导下,我们发现在 bDOT 中引入自由基稳定取代基可以显着提高与甲基丙烯酸甲酯 (MMA) 的可调节共聚反应性。通过分子结构的迭代优化,发现一种特定的bDOT, F - p -CF 3 PhDOT ,可以与MMA有效共聚。使用工业相关的本体自由基共聚条件制备具有低百分比F- p -CF 3 Ph 3 PhDOT (1.8 和 3.8 mol%)的高摩尔质量可解构 PMMA 基共聚物(dPMMA,M n > 120 kDa)。 dPMMA的热机械性能与PMMA相似;然而,前者在温和的氨解条件下会降解成低摩尔质量片段(<6.5 kDa)。这项工作提出了第一个自由基开环 CC 能够与 MMA 几乎无规共聚而无需交联的例子,并为未来可解构共聚物的机制引导设计提供了工作流程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号