当前位置:
X-MOL 学术
›
FEBS Journal
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the peculiarities and development of novel inhibitors of leishmanial arginyl‐tRNA synthetase
FEBS Journal Pub Date : 2024-03-25 , DOI: 10.1111/febs.17122 Fouzia Nasim 1 , Muppidi Shravan Kumar 2 , Mallika Alvala 2 , Insaf Ahmed Qureshi 1
FEBS Journal Pub Date : 2024-03-25 , DOI: 10.1111/febs.17122 Fouzia Nasim 1 , Muppidi Shravan Kumar 2 , Mallika Alvala 2 , Insaf Ahmed Qureshi 1
Affiliation
|
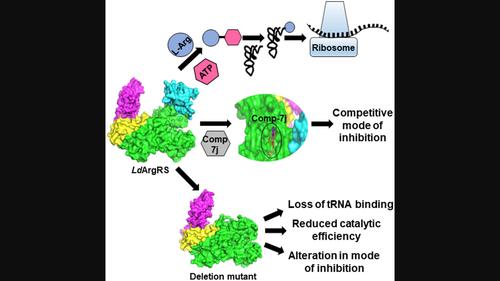
Aminoacylation by tRNA synthetase is a crucial part of protein synthesis and is widely recognized as a therapeutic target for drug development. Unlike the arginyl‐tRNA synthetases (ArgRSs) reported previously, here, we report an ArgRS of Leishmania donovani (Ld ArgRS) that can follow the canonical two‐step aminoacylation process. Since a previously uncharacterized insertion region is present within its catalytic domain, we implemented the splicing by overlap extension PCR (SOE–PCR) method to create a deletion mutant (ΔIns‐Ld ArgRS) devoid of this region to investigate its function. Notably, the purified Ld ArgRS and ΔIns‐Ld ArgRS exhibited different oligomeric states along with variations in their enzymatic activity. The full‐length protein showed better catalytic efficiency than ΔIns‐Ld ArgRS, and the insertion region was identified as the tRNA binding domain. In addition, a benzothiazolo‐coumarin derivative (Comp‐7j) possessing high pharmacokinetic properties was recognized as a competitive and more specific inhibitor of Ld ArgRS than its human counterpart. Removal of the insertion region altered the mode of inhibition for ΔIns‐Ld ArgRS and caused a reduction in the inhibitor's binding affinity. Both purified proteins depicted variances in the secondary structural content upon ligand binding and thus, thermostability. Apart from the trypanosomatid‐specific insertion and Rossmann fold motif, Ld ArgRS revealed typical structural characteristics of ArgRSs, and Comp‐7j was found to bind within the ATP binding pocket. Furthermore, the placement of tRNAArg near the insertion region enhanced the stability and compactness of Ld ArgRS compared to other ligands. This study thus reports a unique ArgRS with respect to catalytic as well as structural properties, which can be considered a plausible drug target for the derivation of novel anti‐leishmanial agents.
中文翻译:

揭示利什曼原虫精氨酰-tRNA合成酶新型抑制剂的特性和开发
tRNA 合成酶的氨酰化是蛋白质合成的关键部分,被广泛认为是药物开发的治疗靶点。与之前报道的精氨酰-tRNA合成酶(ArgRS)不同,在这里,我们报道的ArgRS为杜氏利什曼原虫 (LD ArgRS)可以遵循规范的两步氨酰化过程。由于先前未表征的插入区域存在于其催化结构域内,因此我们通过重叠延伸PCR(SOE-PCR)方法实施了剪接,以创建缺失突变体(ΔIns-LD ArgRS)缺乏该区域来研究其功能。值得注意的是,纯化后的LD ArgRS 和 ΔIns-LD ArgRS 表现出不同的寡聚状态及其酶活性的变化。全长蛋白表现出比 ΔIns- 更好的催化效率LD ArgRS,插入区被鉴定为tRNA结合域。此外,具有高药代动力学特性的苯并噻唑并香豆素衍生物(Comp-7j)被认为是一种竞争性且更特异的抑制剂。LD ArgRS 与人类对应物相比。插入区域的去除改变了 ΔIns- 的抑制模式LD ArgRS 并导致抑制剂的结合亲和力降低。两种纯化的蛋白质都描绘了配体结合后二级结构内容的变化,以及热稳定性的变化。除了锥虫特有的插入和罗斯曼折叠基序之外,LD ArgRS 揭示了 ArgRS 的典型结构特征,并且发现 Comp-7j 结合在 ATP 结合袋内。此外,tRNA 的放置精氨酸 靠近插入区域增强了稳定性和紧凑性LD ArgRS 与其他配体的比较。因此,这项研究报告了一种在催化和结构特性方面独特的 ArgRS,它可以被认为是衍生新型抗利什曼病药物的合理药物靶标。
更新日期:2024-03-25
中文翻译:

揭示利什曼原虫精氨酰-tRNA合成酶新型抑制剂的特性和开发
tRNA 合成酶的氨酰化是蛋白质合成的关键部分,被广泛认为是药物开发的治疗靶点。与之前报道的精氨酰-tRNA合成酶(ArgRS)不同,在这里,我们报道的ArgRS为



























 京公网安备 11010802027423号
京公网安备 11010802027423号