当前位置:
X-MOL 学术
›
FEBS Journal
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel pathological mutant reveals the role of torsional flexibility in the serpin breach in adoption of an aggregation‐prone intermediate
FEBS Journal Pub Date : 2024-03-25 , DOI: 10.1111/febs.17121 Kamila Kamuda 1, 2 , Riccardo Ronzoni 1, 2 , Avik Majumdar 3, 4, 5 , Fiona H. X. Guan 3 , James A. Irving 1, 2 , David A. Lomas 1, 2
FEBS Journal Pub Date : 2024-03-25 , DOI: 10.1111/febs.17121 Kamila Kamuda 1, 2 , Riccardo Ronzoni 1, 2 , Avik Majumdar 3, 4, 5 , Fiona H. X. Guan 3 , James A. Irving 1, 2 , David A. Lomas 1, 2
Affiliation
|
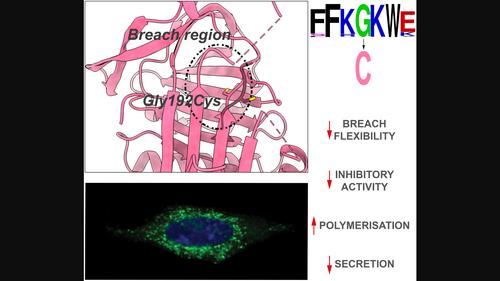
Mutants of alpha‐1‐antitrypsin cause the protein to self‐associate and form ordered aggregates (‘polymers’) that are retained within hepatocytes, resulting in a predisposition to the development of liver disease. The associated reduction in secretion, and for some mutants, impairment of function, leads to a failure to protect lung tissue against proteases released during the inflammatory response and an increased risk of emphysema. We report here a novel deficiency mutation (Gly192Cys), that we name the Sydney variant, identified in a patient in heterozygosity with the Z allele (Glu342Lys). Cellular analysis revealed that the novel variant was mostly retained as insoluble polymers within the endoplasmic reticulum. The basis for this behaviour was investigated using biophysical and structural techniques. The variant showed a 40% reduction in inhibitory activity and a reduced stability as assessed by thermal unfolding experiments. Polymerisation involves adoption of an aggregation‐prone intermediate and paradoxically the energy barrier for transition to this state was increased by 16% for the Gly192Cys variant with respect to the wild‐type protein. However, with activation to the intermediate state, polymerisation occurred at a 3.8‐fold faster rate overall. X‐ray crystallography provided two crystal structures of the Gly192Cys variant, revealing perturbation within the ‘breach’ region with Cys192 in two different orientations: in one structure it faces towards the hydrophobic core while in the second it is solvent‐exposed. This orientational heterogeneity was confirmed by PEGylation. These data show the critical role of the torsional freedom imparted by Gly192 in inhibitory activity and stability against polymerisation.
中文翻译:

一种新的病理突变体揭示了扭转柔性在采用易于聚集的中间体时丝氨酸蛋白酶抑制剂断裂中的作用
α-1-抗胰蛋白酶的突变体导致蛋白质自我缔合并形成保留在肝细胞内的有序聚集体(“聚合物”),从而导致肝病的发生。相关的分泌减少,以及对于某些突变体来说,功能受损,导致无法保护肺组织免受炎症反应期间释放的蛋白酶的影响,并增加肺气肿的风险。我们在此报告了一种新的缺陷突变 (Gly192Cys),我们将其命名为 Sydney 变体,在一名 Z 等位基因 (Glu342Lys) 杂合的患者中发现。细胞分析表明,新变体大部分在内质网内作为不溶性聚合物保留。使用生物物理和结构技术研究了这种行为的基础。通过热解折叠实验评估,该变体的抑制活性降低了 40%,稳定性降低。聚合涉及采用易于聚集的中间体,矛盾的是,相对于野生型蛋白,Gly192Cys 变体过渡到这种状态的能量势垒增加了 16%。然而,随着激活到中间态,聚合反应的总体速度提高了 3.8 倍。 X 射线晶体学提供了 Gly192Cys 变体的两种晶体结构,揭示了 Cys192 在两个不同方向的“突破”区域内的扰动:在一种结构中,它面向疏水核心,而在第二种结构中,它暴露在溶剂中。这种方向异质性通过聚乙二醇化得到证实。这些数据显示了 Gly192 赋予的扭转自由度在抑制活性和聚合稳定性方面的关键作用。
更新日期:2024-03-25
中文翻译:

一种新的病理突变体揭示了扭转柔性在采用易于聚集的中间体时丝氨酸蛋白酶抑制剂断裂中的作用
α-1-抗胰蛋白酶的突变体导致蛋白质自我缔合并形成保留在肝细胞内的有序聚集体(“聚合物”),从而导致肝病的发生。相关的分泌减少,以及对于某些突变体来说,功能受损,导致无法保护肺组织免受炎症反应期间释放的蛋白酶的影响,并增加肺气肿的风险。我们在此报告了一种新的缺陷突变 (Gly192Cys),我们将其命名为 Sydney 变体,在一名 Z 等位基因 (Glu342Lys) 杂合的患者中发现。细胞分析表明,新变体大部分在内质网内作为不溶性聚合物保留。使用生物物理和结构技术研究了这种行为的基础。通过热解折叠实验评估,该变体的抑制活性降低了 40%,稳定性降低。聚合涉及采用易于聚集的中间体,矛盾的是,相对于野生型蛋白,Gly192Cys 变体过渡到这种状态的能量势垒增加了 16%。然而,随着激活到中间态,聚合反应的总体速度提高了 3.8 倍。 X 射线晶体学提供了 Gly192Cys 变体的两种晶体结构,揭示了 Cys192 在两个不同方向的“突破”区域内的扰动:在一种结构中,它面向疏水核心,而在第二种结构中,它暴露在溶剂中。这种方向异质性通过聚乙二醇化得到证实。这些数据显示了 Gly192 赋予的扭转自由度在抑制活性和聚合稳定性方面的关键作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号