当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Advancing Natural Product Discovery: A Structure-Oriented Fractions Screening Platform for Compound Annotation and Isolation
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-24 , DOI: 10.1021/acs.analchem.3c05057 Yichao Ge 1, 2 , Chengzeng Zhou 1 , Yihan Ma 1 , Zihan Wang 3 , Shufan Wang 4 , Wei Wang 3 , Bin Wu 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-24 , DOI: 10.1021/acs.analchem.3c05057 Yichao Ge 1, 2 , Chengzeng Zhou 1 , Yihan Ma 1 , Zihan Wang 3 , Shufan Wang 4 , Wei Wang 3 , Bin Wu 1
Affiliation
|
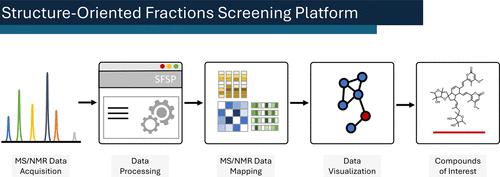
Natural product discovery is hindered by the lack of tools that integrate untargeted nuclear magnetic resonance and mass spectrometry data on a library scale. This article describes the first application of the innovative NMR/MS-based machine learning tool, the “Structure-Oriented Fractions Screening Platform (SFSP)”, enabling functional-group-guided fractionation and accelerating the discovery and characterization of undescribed natural products. The concept was applied to the extract of a marine fungus known to be a prolific producer of diverse natural products. With the assistance of SFSP, we isolated 24 flavipidin derivatives and five phenalenone analogues from Aspergillus sp. GE2-6, revealing 27 undescribed compounds. Compounds 7–22 were proposed as isomeric derivatives featuring a 5/6-ring fusion, formed by the dimerization of flavipidin E (5). Compounds 23 and 24 were envisaged as isomeric derivatives with a 6/5/6-ring fusion, generated through the degradation of two flavipidin E molecules. Furthermore, flavipidin A (1) and asperphenalenone E (28) exhibited potent anti-influenza (PR8) activities, with IC50 values of 21.9 ± 0.2 and 12.9 ± 0.1 μM, respectively. Meanwhile, asperphenalenone (26) and asperphenalenone P (27) treatments exhibited significant inhibition of HIV pseudovirus infection in 293FT cells, boasting IC50 values of 6.1 ± 0.9 and 4.6 ± 1.1 μM, respectively. Overall, SFSP streamlines natural product isolation through NMR and MS data integration, as showcased by the discovery of numerous undescribed flavipidins and phenalenones based on NMR olefinic signals and low-field hydroxy signals.
中文翻译:

推进天然产物发现:用于化合物注释和分离的面向结构的分数筛选平台
由于缺乏在图书馆规模上整合非靶向核磁共振和质谱数据的工具,天然产物的发现受到阻碍。本文介绍了基于 NMR/MS 的创新机器学习工具“面向结构的馏分筛选平台 (SFSP)”的首次应用,该工具可实现官能团引导的分馏并加速未描述的天然产物的发现和表征。这一概念被应用于一种海洋真菌的提取物中,这种真菌是多种天然产品的多产者。在SFSP的帮助下,我们从曲霉菌中分离出了24种黄维啶衍生物和5种苯烯酮类似物。 GE2-6,揭示了 27 种未描述的化合物。化合物7 – 22被认为是具有 5/6 环融合特征的异构衍生物,由 flavipidin E 二聚形成 ( 5 )。化合物23和24被认为是具有 6/5/6 环融合的异构体衍生物,通过两个 flavipidin E 分子的降解产生。此外,flavipidin A ( 1 ) 和 asperphenalenone E ( 28 ) 表现出有效的抗流感 (PR8) 活性,IC 50值分别为 21.9 ± 0.2 和 12.9 ± 0.1 μM。同时,Asperphenalenone ( 26 ) 和 Asperphenalenone P ( 27 ) 治疗对 293FT 细胞中的 HIV 假病毒感染表现出显着抑制作用,IC 50值分别为 6.1 ± 0.9 和 4.6 ± 1.1 μM。总体而言,SFSP 通过 NMR 和 MS 数据集成简化了天然产物的分离,基于 NMR 烯烃信号和低场羟基信号发现了大量未描述的黄维啶和苯烯酮就证明了这一点。
更新日期:2024-03-24
中文翻译:

推进天然产物发现:用于化合物注释和分离的面向结构的分数筛选平台
由于缺乏在图书馆规模上整合非靶向核磁共振和质谱数据的工具,天然产物的发现受到阻碍。本文介绍了基于 NMR/MS 的创新机器学习工具“面向结构的馏分筛选平台 (SFSP)”的首次应用,该工具可实现官能团引导的分馏并加速未描述的天然产物的发现和表征。这一概念被应用于一种海洋真菌的提取物中,这种真菌是多种天然产品的多产者。在SFSP的帮助下,我们从曲霉菌中分离出了24种黄维啶衍生物和5种苯烯酮类似物。 GE2-6,揭示了 27 种未描述的化合物。化合物7 – 22被认为是具有 5/6 环融合特征的异构衍生物,由 flavipidin E 二聚形成 ( 5 )。化合物23和24被认为是具有 6/5/6 环融合的异构体衍生物,通过两个 flavipidin E 分子的降解产生。此外,flavipidin A ( 1 ) 和 asperphenalenone E ( 28 ) 表现出有效的抗流感 (PR8) 活性,IC 50值分别为 21.9 ± 0.2 和 12.9 ± 0.1 μM。同时,Asperphenalenone ( 26 ) 和 Asperphenalenone P ( 27 ) 治疗对 293FT 细胞中的 HIV 假病毒感染表现出显着抑制作用,IC 50值分别为 6.1 ± 0.9 和 4.6 ± 1.1 μM。总体而言,SFSP 通过 NMR 和 MS 数据集成简化了天然产物的分离,基于 NMR 烯烃信号和低场羟基信号发现了大量未描述的黄维啶和苯烯酮就证明了这一点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号