当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Shapeshifting Nucleophiles HO–(NH3)n React with Methyl Chloride
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jpca.3c07553 Xiangyu Wu 1 , Yang Hu 1 , Shaowen Zhang 1 , Jing Xie 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jpca.3c07553 Xiangyu Wu 1 , Yang Hu 1 , Shaowen Zhang 1 , Jing Xie 1
Affiliation
|
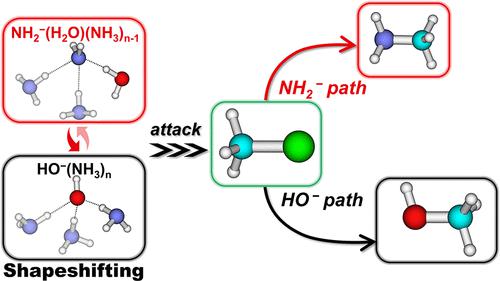
The microsolvated anions HO–(NH3)n were found to induce new nucleophile NH2–(H2O)(NH3)n−1 via intramolecular proton transfer. Hence, the ion–molecule nucleophilic substitution (SN2) reaction between CH3Cl and these shapeshifting nucleophiles lead to both the HO– path and NH2– path, meaning that the respective attacking nucleophile is HO– or NH2–. The CCSD(T) level of calculation was performed to characterize the potential energy surfaces. Calculations indicate that the HO– species are lower in energy than the NH2– species, and the SN2 reaction barriers are lower for the HO– path than the NH2–-path. Incremental solvation increases the barrier for both paths. Comparison between HO–(NH3)n and HOO–(NH3)n confirmed the existence of an α-effect under microsolvated conditions. Comparison between HO–(NH3)n and HO–(H2O)n indicated that the more polarized H2O stabilizes the nucleophiles more than NH3, and thus, the hydrated systems have higher SN2 reaction barriers. The aforementioned barrier changes can be explained by the differential stabilization of the nucleophile and HOMO levels upon solvation, thus affecting the HOMO–LUMO interaction between the nucleophile and substrate. For the same kind of nucleophilic attacking atom, O or N, the reaction barrier has a good linear correlation with the HOMO level of the nucleophiles. Hence, the HOMO level or the binding energy of microsolvated nucleophiles is a good indicator to evaluate the order of barrier heights. This work expands our understanding of the microsolvation effect on prototype SN2 reactions beyond the water solvent.
中文翻译:

变形亲核试剂 HO–(NH3)n 与氯甲烷反应
发现微溶剂化阴离子HO – (NH 3 ) n通过分子内质子转移诱导新的亲核试剂NH 2 – (H 2 O)(NH 3 ) n -1 。因此, CH 3 Cl和这些变形亲核试剂之间的离子-分子亲核取代(S N 2)反应导致HO -路径和NH 2 -路径,这意味着各自的攻击亲核试剂是HO -或NH 2 -。执行 CCSD(T) 级别的计算来表征势能面。计算表明,HO -物质的能量低于NH 2 -物质,并且HO -路径的S N 2 反应势垒低于NH 2 -路径。增量溶剂化增加了两条路径的障碍。 HO – (NH 3 ) n和HOO – (NH 3 ) n之间的比较证实了微溶剂化条件下α效应的存在。 HO – (NH 3 ) n和HO – (H 2 O) n之间的比较表明,极化程度更高的H 2 O比NH 3更能稳定亲核试剂,因此水合体系具有更高的S N 2 反应势垒。上述势垒变化可以通过溶剂化时亲核试剂和 HOMO 水平的差异稳定来解释,从而影响亲核试剂和底物之间的 HOMO-LUMO 相互作用。对于同一类亲核攻击原子O或N,反应势垒与亲核体的HOMO能级具有良好的线性相关性。因此,HOMO能级或微溶剂化亲核试剂的结合能是评估势垒高度顺序的良好指标。这项工作扩展了我们对水溶剂之外的原型 S N 2 反应的微溶剂化效应的理解。
更新日期:2024-03-26
中文翻译:

变形亲核试剂 HO–(NH3)n 与氯甲烷反应
发现微溶剂化阴离子HO – (NH 3 ) n通过分子内质子转移诱导新的亲核试剂NH 2 – (H 2 O)(NH 3 ) n -1 。因此, CH 3 Cl和这些变形亲核试剂之间的离子-分子亲核取代(S N 2)反应导致HO -路径和NH 2 -路径,这意味着各自的攻击亲核试剂是HO -或NH 2 -。执行 CCSD(T) 级别的计算来表征势能面。计算表明,HO -物质的能量低于NH 2 -物质,并且HO -路径的S N 2 反应势垒低于NH 2 -路径。增量溶剂化增加了两条路径的障碍。 HO – (NH 3 ) n和HOO – (NH 3 ) n之间的比较证实了微溶剂化条件下α效应的存在。 HO – (NH 3 ) n和HO – (H 2 O) n之间的比较表明,极化程度更高的H 2 O比NH 3更能稳定亲核试剂,因此水合体系具有更高的S N 2 反应势垒。上述势垒变化可以通过溶剂化时亲核试剂和 HOMO 水平的差异稳定来解释,从而影响亲核试剂和底物之间的 HOMO-LUMO 相互作用。对于同一类亲核攻击原子O或N,反应势垒与亲核体的HOMO能级具有良好的线性相关性。因此,HOMO能级或微溶剂化亲核试剂的结合能是评估势垒高度顺序的良好指标。这项工作扩展了我们对水溶剂之外的原型 S N 2 反应的微溶剂化效应的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号