当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ozonolysis of 2-Methyl-2-pentenal: New Insights from Master Equation Modeling
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jpca.3c04965 Najoua Derbel 1, 2 , Carmen Kalalian 2 , Alexander Alijah 2 , Struan H. Robertson 3 , Abdelkhaleq Chakir 2 , Estelle Roth 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jpca.3c04965 Najoua Derbel 1, 2 , Carmen Kalalian 2 , Alexander Alijah 2 , Struan H. Robertson 3 , Abdelkhaleq Chakir 2 , Estelle Roth 2
Affiliation
|
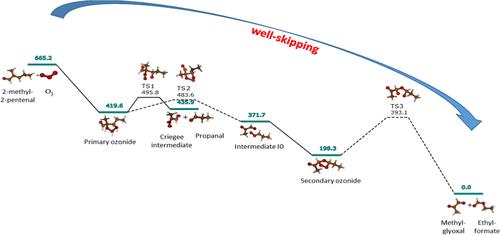
Experimental and theoretical studies were carried out to investigate the ozonolysis of trans-2-methyl-2-pentenal. The experiments were conducted in atmospheric simulation chambers coupled to a Fourier transform infrared (FTIR) spectrometer and a gas chromatograph–mass spectrometer at room temperature and atmospheric pressure in the presence of an excess of cyclohexane in dry conditions (RH < 1%). The ozonolysis reaction was investigated theoretically from the results of accurate density functional (M06-2X) and ab initio [CCSD(T)] computations, employing the AVTZ basis set. The sequence of reaction steps was established, and the system of kinetics equations was modeled using MESMER. In the first step, a primary ozonide is formed, which then decomposes along two pathways. The principal ozonolysis products are propanal, methylglyoxal, ethylformate, and a secondary ozonide. An interesting competition between sequential reaction steps and well-skipping is found, which leads to an inversion of the expected methylglyoxal/propanal product ratio at temperatures below 210 K. The mechanism of the “hot ester” reaction channel of the Criegee intermediate was revisited. The computed ozonolysis rate constant and product branching ratio are in excellent agreement with the experimental data that are also reported in the present work.
中文翻译:

2-甲基-2-戊烯醛的臭氧分解:主方程建模的新见解
进行了实验和理论研究来研究反式-2-甲基-2-戊烯醛的臭氧分解。实验在大气模拟室中进行,并配有傅里叶变换红外 (FTIR) 光谱仪和气相色谱-质谱仪,在室温和大气压下,在干燥条件下(相对湿度 < 1%)存在过量环己烷。采用 AVTZ 基组,根据精确密度泛函 (M06-2X) 和从头计算 [CCSD(T)] 计算的结果,从理论上研究了臭氧分解反应。建立了反应步骤顺序,并使用 MESMER 建立了动力学方程组模型。第一步,形成初级臭氧化物,然后沿着两条路径分解。主要的臭氧分解产物是丙醛、甲基乙二醛、甲酸乙酯和次级臭氧化物。发现连续反应步骤和跳孔之间存在有趣的竞争,这导致在低于 210 K 的温度下预期的甲基乙二醛/丙醛产物比例发生反转。重新审视了 Criegee 中间体的“热酯”反应通道的机制。计算的臭氧分解速率常数和产物支化比与本工作中报告的实验数据非常一致。
更新日期:2024-03-26
中文翻译:

2-甲基-2-戊烯醛的臭氧分解:主方程建模的新见解
进行了实验和理论研究来研究反式-2-甲基-2-戊烯醛的臭氧分解。实验在大气模拟室中进行,并配有傅里叶变换红外 (FTIR) 光谱仪和气相色谱-质谱仪,在室温和大气压下,在干燥条件下(相对湿度 < 1%)存在过量环己烷。采用 AVTZ 基组,根据精确密度泛函 (M06-2X) 和从头计算 [CCSD(T)] 计算的结果,从理论上研究了臭氧分解反应。建立了反应步骤顺序,并使用 MESMER 建立了动力学方程组模型。第一步,形成初级臭氧化物,然后沿着两条路径分解。主要的臭氧分解产物是丙醛、甲基乙二醛、甲酸乙酯和次级臭氧化物。发现连续反应步骤和跳孔之间存在有趣的竞争,这导致在低于 210 K 的温度下预期的甲基乙二醛/丙醛产物比例发生反转。重新审视了 Criegee 中间体的“热酯”反应通道的机制。计算的臭氧分解速率常数和产物支化比与本工作中报告的实验数据非常一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号