当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multi-site catalysis of high-entropy hydroxides for sustainable electrooxidation of glucose to glucaric acid
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-03-26 , DOI: 10.1039/d4ee00221k Xianhong Wu 1, 2 , Zhi-Jian Zhao 3 , Xiangcheng Shi 3, 4 , Liqun Kang 5 , Pratteek Das 1 , Sen Wang 1 , Shengqi Chu 6 , Hua Wang 7 , Kenneth Davey 8 , Bo Zhang 7 , Shi-Zhang Qiao 8 , Jinlong Gong 3 , Zhong-Shuai Wu 1, 9
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-03-26 , DOI: 10.1039/d4ee00221k Xianhong Wu 1, 2 , Zhi-Jian Zhao 3 , Xiangcheng Shi 3, 4 , Liqun Kang 5 , Pratteek Das 1 , Sen Wang 1 , Shengqi Chu 6 , Hua Wang 7 , Kenneth Davey 8 , Bo Zhang 7 , Shi-Zhang Qiao 8 , Jinlong Gong 3 , Zhong-Shuai Wu 1, 9
Affiliation
|
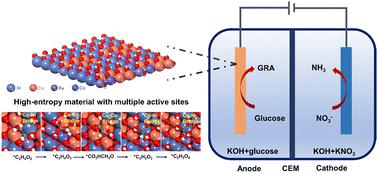
The glucose electrooxidation reaction (GOR) is an environmentally benign way to generate high value-add glucaric acid. However, a lack of suitable catalysts for the GOR limits development. Here we report for the first time a practically robust, multi-site, synergistic catalyst of defect-rich high-entropy FeCoNiCu layered double-hydroxide nanosheets grown on nickel foam. We demonstrate a highly significant activity and stability for the GOR leading to a low potential of 1.22 V vs. RHE at a current density of 100 mA cm−2, together with an excellent glucose conversion of ∼100% and glucaric acid yield of >90%. We evidence that the Cu–Co bridge promotes dehydrogenation of the hydroxyl group, and that the Cu–Cu bridge boosts dehydrogenation on carbon to form an aldehyde group. We establish that the Cu–Ni bridge boosts the oxidation of the aldehyde group to carboxyl to exhibit an important advantage of multi-site synergistic catalysis of high entropy hydroxides. We confirm an energy-saving hybrid flow electrolytic cell, prototype coupled GOR with a nitrate reduction reaction (NO3−RR), that requires an applied voltage of just 1.07 and 1.32 V for an electrolytic current density of, respectively, 10 and 100 mA cm−2 for GOR‖NO3−RR, together with concurrent low-potential production of glucaric acid and NH3. We conclude that defect-rich high-entropy FeCoNiCu catalysis of high-entropy hydroxides can be used for the practical design of sustainable and environmentally benign electrooxidation of glucose to glucaric acid. Our findings will be of benefit to researchers and manufacturers in the electrocatalytic conversion of renewable biomass for high value-add chemicals.
中文翻译:

高熵氢氧化物的多位点催化将葡萄糖可持续电氧化为葡萄糖二酸
葡萄糖电氧化反应(GOR)是一种生产高附加值葡萄糖酸的环境友好方法。然而,GOR 缺乏合适的催化剂限制了其发展。在这里,我们首次报道了一种在泡沫镍上生长的、富含缺陷的高熵 FeCoNiCu 层状双氢氧化物纳米片的实用稳健的多位点协同催化剂。我们证明了 GOR 具有非常显着的活性和稳定性,导致在 100 mA cm -2的电流密度下相对于RHE 具有 1.22 V 的低电势,同时具有出色的葡萄糖转化率 ∼100% 和葡萄糖二酸产率 >90 %。我们证明 Cu-Co 桥促进羟基脱氢,并且 Cu-Cu 桥促进碳脱氢形成醛基。我们发现,Cu-Ni桥促进醛基氧化成羧基,从而展现出高熵氢氧化物多位点协同催化的重要优势。我们确认了一种节能混合流电解池,其原型结合了 GOR 和硝酸盐还原反应 (NO 3 − RR),仅需 1.07 和 1.32 V 的外加电压即可实现分别为 10 和 100 mA 的电解电流密度cm -2对于GOR‖NO 3 − RR,同时产生葡萄糖二酸和NH 3的低潜力。我们得出的结论是,高熵氢氧化物的富缺陷高熵 FeCoNiCu 催化可用于葡萄糖可持续且环境友好的电氧化生成葡萄糖酸的实际设计。我们的研究结果将有利于研究人员和制造商将可再生生物质电催化转化为高附加值化学品。
更新日期:2024-03-26
中文翻译:

高熵氢氧化物的多位点催化将葡萄糖可持续电氧化为葡萄糖二酸
葡萄糖电氧化反应(GOR)是一种生产高附加值葡萄糖酸的环境友好方法。然而,GOR 缺乏合适的催化剂限制了其发展。在这里,我们首次报道了一种在泡沫镍上生长的、富含缺陷的高熵 FeCoNiCu 层状双氢氧化物纳米片的实用稳健的多位点协同催化剂。我们证明了 GOR 具有非常显着的活性和稳定性,导致在 100 mA cm -2的电流密度下相对于RHE 具有 1.22 V 的低电势,同时具有出色的葡萄糖转化率 ∼100% 和葡萄糖二酸产率 >90 %。我们证明 Cu-Co 桥促进羟基脱氢,并且 Cu-Cu 桥促进碳脱氢形成醛基。我们发现,Cu-Ni桥促进醛基氧化成羧基,从而展现出高熵氢氧化物多位点协同催化的重要优势。我们确认了一种节能混合流电解池,其原型结合了 GOR 和硝酸盐还原反应 (NO 3 − RR),仅需 1.07 和 1.32 V 的外加电压即可实现分别为 10 和 100 mA 的电解电流密度cm -2对于GOR‖NO 3 − RR,同时产生葡萄糖二酸和NH 3的低潜力。我们得出的结论是,高熵氢氧化物的富缺陷高熵 FeCoNiCu 催化可用于葡萄糖可持续且环境友好的电氧化生成葡萄糖酸的实际设计。我们的研究结果将有利于研究人员和制造商将可再生生物质电催化转化为高附加值化学品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号