当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dual-anion chemistry synchronously regulating the solvation structure and electric double layer for durable Zn metal anodes
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-03-26 , DOI: 10.1039/d4ee00109e Rong Huang 1 , Jingwei Zhang 1 , Wei Wang 1 , Xiaohong Wu 2 , Xuelong Liao 1 , Tiantian Lu 1 , Youzeng Li 1 , Jialei Chen 1 , Shan Chen 1 , Yu Qiao 2 , Qing Zhao 1 , Huan Wang 1
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2024-03-26 , DOI: 10.1039/d4ee00109e Rong Huang 1 , Jingwei Zhang 1 , Wei Wang 1 , Xiaohong Wu 2 , Xuelong Liao 1 , Tiantian Lu 1 , Youzeng Li 1 , Jialei Chen 1 , Shan Chen 1 , Yu Qiao 2 , Qing Zhao 1 , Huan Wang 1
Affiliation
|
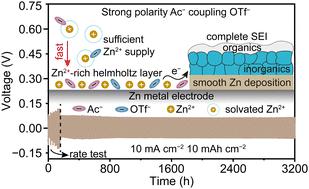
The zinc (Zn) metal electrode suffers from poor stability especially at high currents and large capacities due to insufficient Zn2+ supply and intricate side reactions. Despite significant progress, the fundamental understanding of the correlation between anions and the induced electrode/electrolyte interface remains elusive. Here, a comparative framework based on anion-polarity hybridization is constructed, generating variations of regulation in the bulk solvation and interfacial structure. Herein, a dual-anion electrolyte towards the durable Zn electrode is modulated by incorporating the strong-polarity acetate anion (Ac−) together with the trifluoromethanesulfonate anion (OTf−), which synchronously prompts anion enrichment in the solvation structure and abundant Zn2+ aggregation in the Helmholtz layer. Remarkably, a cumulative plating capacity of 15.25 A h cm−2 (3050 h) is harvested in the Zn‖Zn symmetric cell at 10 mA cm−2 and 10 mA h cm−2. Moreover, the designed electrolyte demonstrates superior adaptability to varying low temperatures and high temperature (60 °C). Zn-ion hybrid capacitors and Zn–air batteries also manifest enhanced electrochemical performance, demonstrating the feasibility of dual-anion chemistry in various electrochemical devices. This study provides the fundamental principle to construct advanced electrolytes via anion chemistry for high-performance Zn-based electrochemical devices.
中文翻译:

双阴离子化学同步调节溶剂化结构和双电层以获得耐用的锌金属阳极
由于Zn 2+供应不足和复杂的副反应,锌(Zn)金属电极的稳定性较差,特别是在高电流和大容量时。尽管取得了重大进展,但对阴离子与感应电极/电解质界面之间的相关性的基本理解仍然难以捉摸。在这里,构建了基于阴离子极性杂化的比较框架,在本体溶剂化和界面结构中产生调节变化。在此,通过将强极性乙酸根阴离子(Ac -)与三氟甲磺酸根阴离子(OTf -)结合来调制面向耐用锌电极的双阴离子电解质,这同时促进了溶剂化结构中的阴离子富集和丰富的Zn 2+亥姆霍兹层中的聚集。值得注意的是, Zn‖Zn对称电池在10 mA cm -2和10 mA h cm -2下获得了15.25 A h cm -2 (3050 h)的累积电镀容量。此外,设计的电解质表现出对不同低温和高温(60°C)的卓越适应性。锌离子混合电容器和锌空气电池也表现出增强的电化学性能,证明了双阴离子化学在各种电化学装置中的可行性。这项研究为通过阴离子化学构建高性能锌基电化学装置的先进电解质提供了基本原理。
更新日期:2024-03-26
中文翻译:

双阴离子化学同步调节溶剂化结构和双电层以获得耐用的锌金属阳极
由于Zn 2+供应不足和复杂的副反应,锌(Zn)金属电极的稳定性较差,特别是在高电流和大容量时。尽管取得了重大进展,但对阴离子与感应电极/电解质界面之间的相关性的基本理解仍然难以捉摸。在这里,构建了基于阴离子极性杂化的比较框架,在本体溶剂化和界面结构中产生调节变化。在此,通过将强极性乙酸根阴离子(Ac -)与三氟甲磺酸根阴离子(OTf -)结合来调制面向耐用锌电极的双阴离子电解质,这同时促进了溶剂化结构中的阴离子富集和丰富的Zn 2+亥姆霍兹层中的聚集。值得注意的是, Zn‖Zn对称电池在10 mA cm -2和10 mA h cm -2下获得了15.25 A h cm -2 (3050 h)的累积电镀容量。此外,设计的电解质表现出对不同低温和高温(60°C)的卓越适应性。锌离子混合电容器和锌空气电池也表现出增强的电化学性能,证明了双阴离子化学在各种电化学装置中的可行性。这项研究为通过阴离子化学构建高性能锌基电化学装置的先进电解质提供了基本原理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号