当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Li+ and Mg2+ Diffusion at the Polymer–Ionic Liquid Interface within PVDF‐Based Ionogel Electrolytes for Batteries and Metal‐Ion Capacitors
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2024-03-27 , DOI: 10.1002/aenm.202304342 Nicolas Demarthe 1, 2, 3 , Luke A. O'Dell 4 , Bernard Humbert 1 , R. Dario Arrua 2 , Drew Evans 2 , Thierry Brousse 1, 3 , Jean Le Bideau 1, 3
Advanced Energy Materials ( IF 27.8 ) Pub Date : 2024-03-27 , DOI: 10.1002/aenm.202304342 Nicolas Demarthe 1, 2, 3 , Luke A. O'Dell 4 , Bernard Humbert 1 , R. Dario Arrua 2 , Drew Evans 2 , Thierry Brousse 1, 3 , Jean Le Bideau 1, 3
Affiliation
|
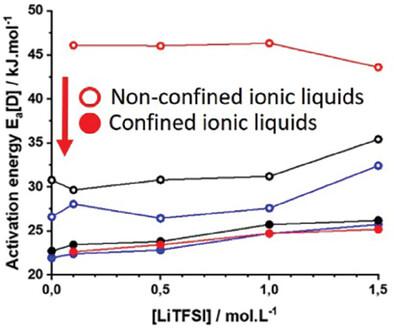
With the widespread use of batteries, their increased performance is of growing in importance. One avenue for this is the enhancement of ion diffusion, particularly for solid‐state electrolytes, for different ions such as lithium (Li+ ) and magnesium (Mg2+ ). Unraveling the origin of better cation diffusion in confined ionic liquids (ILs) in a polymer matrix (ionogels) is compared to that of the IL itself. Ionic conductivity measured by electrochemical impedance spectroscopy for ionogels (7.0 mS cm−1 at 30 °C) is very close to the conductivity of the non‐confined IL (8.9 mS cm−1 at 30 °C), that is, 1‐ethyl‐3‐methyimidazolium bis(trifluorosulfonyl)imide (EMIM TFSI). An even better ionic conductivity is observed for confined EMIM TFSI with high concentrations (1 m ) of lithium or magnesium salt added. The improved macroscopic transport properties can be explained by the higher self‐diffusion of each ion at the liquid‐to‐solid interface induced by the confinement in a poly‐vinylidenedifluoride (PVDF) polymer matrix. Upon confinement, the strong breaking down of ion aggregates enables a better diffusion, especially for TFSI anion and strongly polarizing cations (e.g., Li+ , Mg2+ .). The coordination number of these cations in the liquid phase confirmed that Li+ and Mg2+ interact with the polymer matrix. Moreover, it is a major result that the activation energy for diffusion is lowered.
中文翻译:

用于电池和金属离子电容器的 PVDF 基离子凝胶电解质中聚合物-离子液体界面处增强的 Li+ 和 Mg2+ 扩散
随着电池的广泛使用,其性能的提高变得越来越重要。实现这一目标的一种途径是增强离子扩散,特别是对于固态电解质,对于不同的离子,例如锂(Li+ )和镁(Mg2+ )。将聚合物基质(离子凝胶)中的受限离子液体 (IL) 与 IL 本身进行比较,揭示其更好的阳离子扩散的起源。通过电化学阻抗谱测量离子凝胶的离子电导率 (7.0 mS cm−1 30 °C 时)非常接近非约束 IL 的电导率(8.9 mS cm−1 30℃),即1-乙基-3-甲基咪唑鎓双(三氟磺酰基)亚胺(EMIM TFSI)。对于高浓度(1米 )添加的锂盐或镁盐。宏观输运特性的改善可以通过聚偏二氟乙烯(PVDF)聚合物基质中的限制引起的液-固界面上每个离子更高的自扩散来解释。限制后,离子聚集体的强烈分解能够实现更好的扩散,特别是对于 TFSI 阴离子和强极化阳离子(例如 Li+ , 镁2+ .)。这些阳离子在液相中的配位数证实了Li+ 和镁2+ 与聚合物基质相互作用。此外,主要的结果是扩散的活化能降低。
更新日期:2024-03-27
中文翻译:

用于电池和金属离子电容器的 PVDF 基离子凝胶电解质中聚合物-离子液体界面处增强的 Li+ 和 Mg2+ 扩散
随着电池的广泛使用,其性能的提高变得越来越重要。实现这一目标的一种途径是增强离子扩散,特别是对于固态电解质,对于不同的离子,例如锂(Li



























 京公网安备 11010802027423号
京公网安备 11010802027423号