当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the full range of N⋯I⋯X halogen-bonding interactions within a single compound using pressure
Chemical Communications ( IF 4.9 ) Pub Date : 2024-03-27 , DOI: 10.1039/d4cc00847b Richard H. Jones 1 , Craig L. Bull 2, 3 , Nicholas P. Funnell 2 , Kevin S. Knight 4, 5 , William G. Marshall 2
Chemical Communications ( IF 4.9 ) Pub Date : 2024-03-27 , DOI: 10.1039/d4cc00847b Richard H. Jones 1 , Craig L. Bull 2, 3 , Nicholas P. Funnell 2 , Kevin S. Knight 4, 5 , William G. Marshall 2
Affiliation
|
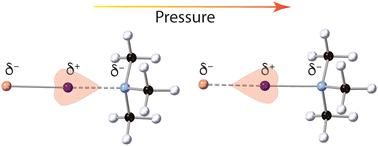
The response of the trimethylammonium–iodinechloride and diiodide (TMA–ICl/I2) crystal structures have been examined under high pressure using neutron powder diffraction. TMA–ICl exhibits impressive pressure-driven electronic flexibility, where the N⋯I–Cl interactions progressively encompass all the distances represented in analogous structures recorded in the Cambridge Structural Database. Comparison with the TMA–I2 complex reveals that this flexibility is owed to the electronegativity of the chlorine atom which induces increased distortion of the iodine electron cloud. This structural flexibility may be influential in the future design of functional molecular materials.
中文翻译:

利用压力探索单一化合物内全方位的 N⋯I⋯X 卤素键相互作用
使用中子粉末衍射在高压下检查了三甲基铵-氯化碘和二碘化物 (TMA-ICl/I 2 ) 晶体结构的响应。 TMA-ICl 表现出令人印象深刻的压力驱动电子灵活性,其中 N⋯I-Cl 相互作用逐渐涵盖剑桥结构数据库中记录的类似结构中表示的所有距离。与TMA-I 2配合物的比较表明,这种灵活性归因于氯原子的电负性,它会导致碘电子云的畸变增加。这种结构灵活性可能会对未来功能分子材料的设计产生影响。
更新日期:2024-03-27
中文翻译:

利用压力探索单一化合物内全方位的 N⋯I⋯X 卤素键相互作用
使用中子粉末衍射在高压下检查了三甲基铵-氯化碘和二碘化物 (TMA-ICl/I 2 ) 晶体结构的响应。 TMA-ICl 表现出令人印象深刻的压力驱动电子灵活性,其中 N⋯I-Cl 相互作用逐渐涵盖剑桥结构数据库中记录的类似结构中表示的所有距离。与TMA-I 2配合物的比较表明,这种灵活性归因于氯原子的电负性,它会导致碘电子云的畸变增加。这种结构灵活性可能会对未来功能分子材料的设计产生影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号