当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electronic isomerism in a heterometallic nickel–iron–sulfur cluster models substrate binding and cyanide inhibition of carbon monoxide dehydrogenase
Chemical Science ( IF 8.4 ) Pub Date : 2024-03-27 , DOI: 10.1039/d4sc00023d Luke C. Lewis 1 , José A. Sanabria-Gracia 1 , Yuri Lee 1, 2 , Adam J. Jenkins 1 , Hannah S. Shafaat 1, 2
Chemical Science ( IF 8.4 ) Pub Date : 2024-03-27 , DOI: 10.1039/d4sc00023d Luke C. Lewis 1 , José A. Sanabria-Gracia 1 , Yuri Lee 1, 2 , Adam J. Jenkins 1 , Hannah S. Shafaat 1, 2
Affiliation
|
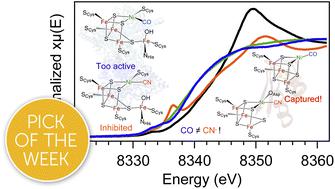
The nickel–iron carbon monoxide dehydrogenase (CODH) enzyme uses a heterometallic nickel–iron–sulfur ([NiFe4S4]) cluster to catalyze the reversible interconversion of carbon dioxide (CO2) and carbon monoxide (CO). These reactions are essential for maintaining the global carbon cycle and offer a route towards sustainable greenhouse gas conversion but have not been successfully replicated in synthetic models, in part due to a poor understanding of the natural system. Though the general protein architecture of CODH is known, the electronic structure of the active site is not well-understood, and the mechanism of catalysis remains unresolved. To better understand the CODH enzyme, we have developed a protein-based model containing a heterometallic [NiFe3S4] cluster in the Pyrococcus furiosus (Pf) ferredoxin (Fd). This model binds small molecules such as carbon monoxide and cyanide, analogous to CODH. Multiple redox- and ligand-bound states of [NiFe3S4] Fd (NiFd) have been investigated using a suite of spectroscopic techniques, including resonance Raman, Ni and Fe K-edge X-ray absorption spectroscopy, and electron paramagnetic resonance, to resolve charge and spin delocalization across the cluster, site-specific electron density, and ligand activation. The facile movement of charge through the cluster highlights the fluidity of electron density within iron–sulfur clusters and suggests an electronic basis by which CN− inhibits the native system while the CO-bound state continues to elude isolation in CODH. The detailed characterization of isolable states that are accessible in our CODH model system provides valuable insight into unresolved enzymatic intermediates and offers design principles towards developing functional mimics of CODH.
中文翻译:

异金属镍-铁-硫簇中的电子异构现象模拟了一氧化碳脱氢酶的底物结合和氰化物抑制
镍铁一氧化碳脱氢酶 (CODH) 使用异金属镍铁硫 ([NiFe 4 S 4 ]) 簇催化二氧化碳 (CO 2 ) 和一氧化碳 (CO) 的可逆相互转化。这些反应对于维持全球碳循环至关重要,并提供了实现可持续温室气体转化的途径,但尚未在合成模型中成功复制,部分原因是对自然系统的了解不足。尽管 CODH 的一般蛋白质结构已知,但活性位点的电子结构尚不清楚,催化机制仍未解决。为了更好地了解 CODH 酶,我们开发了一种基于蛋白质的模型,其中包含激烈火球菌( Pf ) 铁氧还蛋白 (Fd)中的异金属 [NiFe 3 S 4 ] 簇。该模型结合一氧化碳和氰化物等小分子,类似于 CODH。 [NiFe 3 S 4 ] Fd (NiFd)的多种氧化还原态和配体结合态已使用一套光谱技术进行了研究,包括共振拉曼、Ni 和 Fe K 边 X 射线吸收光谱以及电子顺磁共振,解决簇内的电荷和自旋离域、位点特异性电子密度和配体激活问题。电荷在团簇中的轻松移动凸显了铁硫团簇内电子密度的流动性,并提出了 CN -抑制天然系统而 CO 结合态继续逃避 CODH 中隔离的电子基础。我们的 CODH 模型系统中可分离状态的详细表征为未解决的酶中间体提供了宝贵的见解,并为开发 CODH 的功能模拟物提供了设计原则。
更新日期:2024-03-27
中文翻译:

异金属镍-铁-硫簇中的电子异构现象模拟了一氧化碳脱氢酶的底物结合和氰化物抑制
镍铁一氧化碳脱氢酶 (CODH) 使用异金属镍铁硫 ([NiFe 4 S 4 ]) 簇催化二氧化碳 (CO 2 ) 和一氧化碳 (CO) 的可逆相互转化。这些反应对于维持全球碳循环至关重要,并提供了实现可持续温室气体转化的途径,但尚未在合成模型中成功复制,部分原因是对自然系统的了解不足。尽管 CODH 的一般蛋白质结构已知,但活性位点的电子结构尚不清楚,催化机制仍未解决。为了更好地了解 CODH 酶,我们开发了一种基于蛋白质的模型,其中包含激烈火球菌( Pf ) 铁氧还蛋白 (Fd)中的异金属 [NiFe 3 S 4 ] 簇。该模型结合一氧化碳和氰化物等小分子,类似于 CODH。 [NiFe 3 S 4 ] Fd (NiFd)的多种氧化还原态和配体结合态已使用一套光谱技术进行了研究,包括共振拉曼、Ni 和 Fe K 边 X 射线吸收光谱以及电子顺磁共振,解决簇内的电荷和自旋离域、位点特异性电子密度和配体激活问题。电荷在团簇中的轻松移动凸显了铁硫团簇内电子密度的流动性,并提出了 CN -抑制天然系统而 CO 结合态继续逃避 CODH 中隔离的电子基础。我们的 CODH 模型系统中可分离状态的详细表征为未解决的酶中间体提供了宝贵的见解,并为开发 CODH 的功能模拟物提供了设计原则。



























 京公网安备 11010802027423号
京公网安备 11010802027423号