当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unlocking Diverse π-Bond Enrichment Frameworks by the Synthesis and Conversion of Boronated Phenyldiethynylethylenes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-27 , DOI: 10.1021/jacs.4c01989 Jinhui Xie 1 , Wangyang Li 1 , Yong Lu 1 , Yanping Zheng 1 , Yanying Huang 1 , Shanglin Chen 1 , Qiuling Song 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-27 , DOI: 10.1021/jacs.4c01989 Jinhui Xie 1 , Wangyang Li 1 , Yong Lu 1 , Yanping Zheng 1 , Yanying Huang 1 , Shanglin Chen 1 , Qiuling Song 1, 2
Affiliation
|
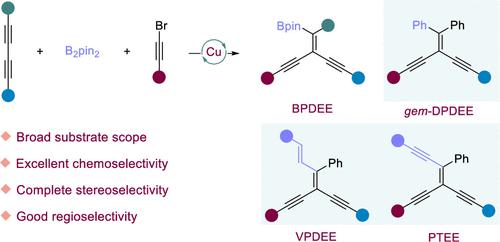
The π-bond enrichment frameworks not only serve as a crucial building block in organic synthesis but also assume a pivotal role in the fields of materials science, biomedicine, photochemistry, and other related disciplines owing to their distinctive structural characteristics. The incorporation of various substituents into the C═C double bonds of tetrasubstituted alkenes is currently a highly significant research area. However, the synthesis of tetrasubstituted alkenes with diverse substituents on double bonds poses a significant challenge in achieving stereoselectivity. Here, we reported an efficient and convergent route of Cu-catalyzed borylalkynylation of both symmetrical and unsymmetrical 1,3-diynes, B2pin2, and acetylene bromide to the construction of boronated phenyldiethynylethylene (BPDEE) derivatives with excellent chemo-, stereo-, and regioselectivities. BPDEE derivatives could transform into novel tetrasubstituted organic π-conjugated gem-diphenyldiethynylethylene (DPDEE), vinylphenyldiethynylethylene (VPDEE), and phenyltriethynylethylene (PTEE) derivatives by a stepwise process, which provides a flexible platform for the synthesis of complex π-bond enrichment frameworks that were difficult to synthesize by previous methods. The initial optical characterization revealed that the synthesized molecules exhibited aggregation-induced emission (AIE) properties, which further establishes the groundwork for future applications and enriches and advances the field of functional π-conjugated frameworks research.
中文翻译:

通过硼化苯基二乙炔基乙烯的合成和转化解锁多种π键富集框架
π键富集框架不仅是有机合成的重要组成部分,而且由于其独特的结构特征,在材料科学、生物医学、光化学和其他相关学科领域中发挥着关键作用。将各种取代基掺入四取代烯烃的C=C双键中是目前非常重要的研究领域。然而,双键上具有不同取代基的四取代烯烃的合成对实现立体选择性提出了重大挑战。在这里,我们报道了一种高效、收敛的铜催化对称和不对称 1,3-二炔、B 2 pin 2和乙炔溴化硼基烷基化反应,构建具有优异化学、立体结构的硼化苯基二乙炔基乙烯 (BPDEE) 衍生物。和区域选择性。 BPDEE衍生物可以通过逐步过程转化为新型四取代有机π-共轭偕二苯基二乙炔乙烯(DPDEE)、乙烯基苯基二乙炔乙烯(VPDEE)和苯基三乙炔乙烯(PTEE)衍生物,这为合成复杂的π键富集框架提供了灵活的平台用以前的方法很难合成。初步的光学表征表明,合成的分子表现出聚集诱导发射(AIE)特性,这进一步为未来的应用奠定了基础,丰富和推进了功能性π共轭框架研究领域。
更新日期:2024-03-27
中文翻译:

通过硼化苯基二乙炔基乙烯的合成和转化解锁多种π键富集框架
π键富集框架不仅是有机合成的重要组成部分,而且由于其独特的结构特征,在材料科学、生物医学、光化学和其他相关学科领域中发挥着关键作用。将各种取代基掺入四取代烯烃的C=C双键中是目前非常重要的研究领域。然而,双键上具有不同取代基的四取代烯烃的合成对实现立体选择性提出了重大挑战。在这里,我们报道了一种高效、收敛的铜催化对称和不对称 1,3-二炔、B 2 pin 2和乙炔溴化硼基烷基化反应,构建具有优异化学、立体结构的硼化苯基二乙炔基乙烯 (BPDEE) 衍生物。和区域选择性。 BPDEE衍生物可以通过逐步过程转化为新型四取代有机π-共轭偕二苯基二乙炔乙烯(DPDEE)、乙烯基苯基二乙炔乙烯(VPDEE)和苯基三乙炔乙烯(PTEE)衍生物,这为合成复杂的π键富集框架提供了灵活的平台用以前的方法很难合成。初步的光学表征表明,合成的分子表现出聚集诱导发射(AIE)特性,这进一步为未来的应用奠定了基础,丰富和推进了功能性π共轭框架研究领域。



























 京公网安备 11010802027423号
京公网安备 11010802027423号