当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Platinum Surface Water Orientation Dictates Hydrogen Evolution Reaction Kinetics in Alkaline Media
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-27 , DOI: 10.1021/jacs.3c12934 Aamir Hassan Shah 1 , Zisheng Zhang 1 , Chengzhang Wan 1, 2 , Sibo Wang 1 , Ao Zhang 2 , Laiyuan Wang 1 , Anastassia N. Alexandrova 1, 3 , Yu Huang 2, 3 , Xiangfeng Duan 1, 3
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2024-03-27 , DOI: 10.1021/jacs.3c12934 Aamir Hassan Shah 1 , Zisheng Zhang 1 , Chengzhang Wan 1, 2 , Sibo Wang 1 , Ao Zhang 2 , Laiyuan Wang 1 , Anastassia N. Alexandrova 1, 3 , Yu Huang 2, 3 , Xiangfeng Duan 1, 3
Affiliation
|
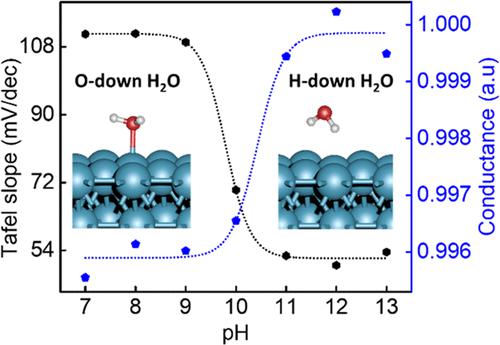
The fundamental understanding of sluggish hydrogen evolution reaction (HER) kinetics on a platinum (Pt) surface in alkaline media is a topic of considerable debate. Herein, we combine cyclic voltammetry (CV) and electrical transport spectroscopy (ETS) approaches to probe the Pt surface at different pH values and develop molecular-level insights into the pH-dependent HER kinetics in alkaline media. The change in HER Tafel slope from ∼110 mV/decade in pH 7–10 to ∼53 mV/decade in pH 11–13 suggests considerably enhanced kinetics at higher pH. The ETS studies reveal a similar pH-dependent switch in the ETS conductance signal at around pH 10, suggesting a notable change of surface adsorbates. Fixed-potential calculations and chemical bonding analysis suggest that this switch is attributed to a change in interfacial water orientation, shifting from primarily an O-down configuration below pH 10 to a H-down configuration above pH 10. This reorientation weakens the O–H bond in the interfacial water molecules and modifies the reaction pathway, leading to considerably accelerated HER kinetics at higher pH. Our integrated studies provide an unprecedented molecular-level understanding of the nontrivial pH-dependent HER kinetics in alkaline media.
中文翻译:

铂表面水取向决定碱性介质中的析氢反应动力学
对碱性介质中铂 (Pt) 表面缓慢析氢反应 (HER) 动力学的基本理解是一个颇具争议的话题。在此,我们结合循环伏安法 (CV) 和电传输光谱 (ETS) 方法来探测不同 pH 值下的 Pt 表面,并在分子水平上深入了解碱性介质中 pH 依赖性 HER 动力学。 HER Tafel 斜率从 pH 7-10 的~110 mV/decade 变化到 pH 11-13 的~53 mV/decade,表明在较高 pH 下动力学显着增强。 ETS 研究表明,在 pH 10 左右,ETS 电导信号存在类似的 pH 依赖性开关,表明表面吸附物发生显着变化。固定电势计算和化学键分析表明,这种转变归因于界面水取向的变化,从主要是低于pH 10的O-向下构型转变为高于pH 10的H-向下构型。这种重新取向削弱了O-H界面水分子中的键合并改变反应途径,导致在较高 pH 下显着加速 HER 动力学。我们的综合研究为碱性介质中非平凡的 pH 依赖性 HER 动力学提供了前所未有的分子水平理解。
更新日期:2024-03-27
中文翻译:

铂表面水取向决定碱性介质中的析氢反应动力学
对碱性介质中铂 (Pt) 表面缓慢析氢反应 (HER) 动力学的基本理解是一个颇具争议的话题。在此,我们结合循环伏安法 (CV) 和电传输光谱 (ETS) 方法来探测不同 pH 值下的 Pt 表面,并在分子水平上深入了解碱性介质中 pH 依赖性 HER 动力学。 HER Tafel 斜率从 pH 7-10 的~110 mV/decade 变化到 pH 11-13 的~53 mV/decade,表明在较高 pH 下动力学显着增强。 ETS 研究表明,在 pH 10 左右,ETS 电导信号存在类似的 pH 依赖性开关,表明表面吸附物发生显着变化。固定电势计算和化学键分析表明,这种转变归因于界面水取向的变化,从主要是低于pH 10的O-向下构型转变为高于pH 10的H-向下构型。这种重新取向削弱了O-H界面水分子中的键合并改变反应途径,导致在较高 pH 下显着加速 HER 动力学。我们的综合研究为碱性介质中非平凡的 pH 依赖性 HER 动力学提供了前所未有的分子水平理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号