当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CH3 Radical Generation in Microplasmas for Up-Conversion of Methane
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jpca.4c00073 Mackenzie Meyer 1 , Sanjana Kerketta 1 , Ryan Hartman 2 , Mark J. Kushner 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-26 , DOI: 10.1021/acs.jpca.4c00073 Mackenzie Meyer 1 , Sanjana Kerketta 1 , Ryan Hartman 2 , Mark J. Kushner 1
Affiliation
|
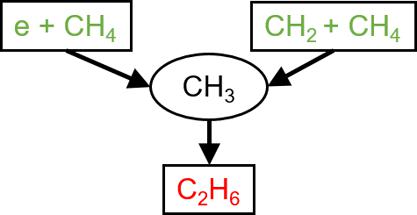
The conversion of methane, CH4, into higher value chemicals using low temperature plasmas is challenged by both improving efficiency and selectivity. One path toward selectivity is capturing plasma-produced methyl radicals, CH3, in a solvent for aqueous processing. Due to the rapid reactions of methyl radicals in the gas phase, the transport distance from the production of the CH3 to its solvation should be short, which then motivates the use of microplasmas. The generation of CH3 in Ar/CH4/H2O plasmas produced in nanosecond pulsed dielectric barrier discharge microplasmas is discussed using results from a computational investigation. The microplasma is sustained in the channel of a microfluidic chip in which the solvent flows along one wall or in droplets. CH3 is primarily produced by electron-impact of and dissociative excitation transfer to CH4, as well as CH2 reacting with CH4. CH3 is rapidly consumed to form C2H6 which, in spite of being subject to these same dissociative processes, accumulates over time, as do other stable products including C3H8 and CH3OH. The gas mixture and electrical properties were varied to assess their effects on CH3 production. CH3 production is largest with 5% CH4 in the Ar/CH4/H2O mixture due to an optimal balance of electron-impact dissociation, which increases with CH4 percentage, and dissociative excitation transfer and CH2 reacting with CH4, which decreases with CH4 percentage. Design parameters of the microchannels were also investigated. Increasing the permittivity of the dielectrics in contact with the plasma increased the ionization wave intensity, which increased CH3 production. Increased energy deposition per pulse generally increases CH3 production as does lengthening pulse length up to a certain point. The arrangement of the solvent flow in the microchannel can also affect the CH3 density and fluence to the solvent. The fluence of CH3 to the liquid solvent is increased if the liquid is immersed in the plasma as a droplet or is a layer on the wall where the ionization wave terminates. The solvation dynamics of CH3 with varying numbers of droplets was also examined. The maximum density of solvated methyl radicals CH3aq occurs with a large number of droplets in the plasma. However, the solvated CH3aq density can rapidly decrease due to desolvation, emphasizing the need to quickly react with the solvated species in the solvent.
中文翻译:

微等离子体中 CH3 自由基的产生用于甲烷的上转换
使用低温等离子体将甲烷CH 4转化为更高价值的化学品面临着提高效率和选择性的挑战。实现选择性的一种途径是在用于水处理的溶剂中捕获等离子体产生的甲基自由基CH 3 。由于甲基自由基在气相中的快速反应,从CH 3的产生到其溶剂化的传输距离应该很短,这促进了微等离子体的使用。使用计算研究的结果讨论了纳秒脉冲介电势垒放电微等离子体中产生的Ar/CH 4 /H 2 O等离子体中CH 3的生成。微等离子体维持在微流控芯片的通道中,其中溶剂沿着壁或以液滴形式流动。 CH 3主要由CH 4的电子撞击和解离激发转移以及CH 2与CH 4反应产生。 CH 3迅速消耗形成C 2 H 6,尽管经历这些相同的解离过程,C 2 H 6 仍随着时间的推移而积累,其他稳定产物包括C 3 H 8和CH 3 OH 也是如此。改变气体混合物和电特性以评估它们对CH 3生产的影响。由于电子碰撞解离的最佳平衡(随着CH 4百分比的增加而增加)、解离激发转移以及CH 2与CH 4反应, CH 3产量最大,Ar/CH 4 /H 2 O混合物中的CH 4为5% ,随着 CH 4百分比的增加而降低。还研究了微通道的设计参数。增加与等离子体接触的电介质的介电常数增加了电离波强度,这增加了CH 3产量。每个脉冲的能量沉积增加通常会增加CH 3产量,将脉冲长度延长到某一点也会增加CH 3 产量。微通道中溶剂流的布置也会影响CH 3密度和对溶剂的注量。如果液体作为液滴浸入等离子体中或者是电离波终止的壁上的层,则CH 3对液体溶剂的注量会增加。还检查了具有不同数量的液滴的CH 3的溶剂化动力学。溶剂化甲基自由基CH的最大密度3aq发生时等离子体中存在大量液滴。然而,溶剂化的CH 3aq密度会由于去溶剂化而迅速降低,这强调了与溶剂中的溶剂化物质快速反应的需要。
更新日期:2024-03-26
中文翻译:

微等离子体中 CH3 自由基的产生用于甲烷的上转换
使用低温等离子体将甲烷CH 4转化为更高价值的化学品面临着提高效率和选择性的挑战。实现选择性的一种途径是在用于水处理的溶剂中捕获等离子体产生的甲基自由基CH 3 。由于甲基自由基在气相中的快速反应,从CH 3的产生到其溶剂化的传输距离应该很短,这促进了微等离子体的使用。使用计算研究的结果讨论了纳秒脉冲介电势垒放电微等离子体中产生的Ar/CH 4 /H 2 O等离子体中CH 3的生成。微等离子体维持在微流控芯片的通道中,其中溶剂沿着壁或以液滴形式流动。 CH 3主要由CH 4的电子撞击和解离激发转移以及CH 2与CH 4反应产生。 CH 3迅速消耗形成C 2 H 6,尽管经历这些相同的解离过程,C 2 H 6 仍随着时间的推移而积累,其他稳定产物包括C 3 H 8和CH 3 OH 也是如此。改变气体混合物和电特性以评估它们对CH 3生产的影响。由于电子碰撞解离的最佳平衡(随着CH 4百分比的增加而增加)、解离激发转移以及CH 2与CH 4反应, CH 3产量最大,Ar/CH 4 /H 2 O混合物中的CH 4为5% ,随着 CH 4百分比的增加而降低。还研究了微通道的设计参数。增加与等离子体接触的电介质的介电常数增加了电离波强度,这增加了CH 3产量。每个脉冲的能量沉积增加通常会增加CH 3产量,将脉冲长度延长到某一点也会增加CH 3 产量。微通道中溶剂流的布置也会影响CH 3密度和对溶剂的注量。如果液体作为液滴浸入等离子体中或者是电离波终止的壁上的层,则CH 3对液体溶剂的注量会增加。还检查了具有不同数量的液滴的CH 3的溶剂化动力学。溶剂化甲基自由基CH的最大密度3aq发生时等离子体中存在大量液滴。然而,溶剂化的CH 3aq密度会由于去溶剂化而迅速降低,这强调了与溶剂中的溶剂化物质快速反应的需要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号