Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Different donors' effect on the electrochemical behavior and electrochromic performances of the D-A type chlorinated polymers
Polymer ( IF 4.6 ) Pub Date : 2024-03-22 , DOI: 10.1016/j.polymer.2024.126943 Daize Mo , Tong Tong , Qingwen Zhang , Qi Feng , Kaiwen Lin
Polymer ( IF 4.6 ) Pub Date : 2024-03-22 , DOI: 10.1016/j.polymer.2024.126943 Daize Mo , Tong Tong , Qingwen Zhang , Qi Feng , Kaiwen Lin
|
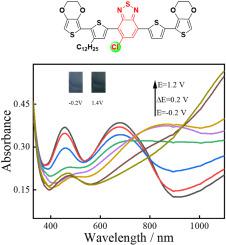
Chlorination has proved to be an efficient method in tuning energy levels and optoelectronic properties of organic semiconducting materials with cheaper synthesis and low-cost raw materials when compared with the fluorinated analogues. Herein, in this work, 5-chlorobenzo[c][1,2,5]thiadizole (Cl-BT) unit was chosen as a new acceptor unit to design two new D-A type electrochromic conjugated polymer: P(Cl-T) and P(Cl-EDOT). The electrochemical behavior and electrochromic performances of above-mentioned chlorinated polymers were studied by electrochemical polymerization techniques and spectroelectrochemistry. Thanks to the strong electron-withdrawing ability of Cl-BT unit and the strong electron-donating properties of EDOT unit, the resultant Cl-EDOT precursor could electropolymerize into high quality hybrid polymer films at lower potential (0.71 V Ag/AgCl) with favorable redox activity and better redox stability. Spectroelectrochemistry shown that as-formed lower band gaps P(Cl-EDOT) exhibits reversible color-changing nature from pale blue to gray accompanied with the moderate optical contrast (13.9%), fast response time (0.6 s), and favorable coloration efficiency (126.4 cm C) in the NIR region that was measured from the kinetic studies. By optimizing the tested electrolyte and solution, its electrochromic performance is further improved (optical contrast: 25.6%, response time: 0.25 s, coloration efficiency: 275.9 C cm). The favorable redox stability and electrochromic performance of P(Cl-EDOT) make it a good candidate to construct electrochromic device, and Cl-BT unit should be a promising building block in designing high performance near infrared electrochromic materials.
中文翻译:

不同给体对DA型氯化聚合物电化学行为和电致变色性能的影响
氯化已被证明是调节有机半导体材料能级和光电性能的有效方法,与氟化类似物相比,其合成成本更低,原材料成本也更低。本工作选择5-氯苯并[c][1,2,5]噻二唑(Cl-BT)单元作为新的受体单元,设计了两种新型DA型电致变色共轭聚合物:P(Cl-T)和P(Cl-EDOT)。采用电化学聚合技术和光谱电化学研究了上述氯化聚合物的电化学行为和电致变色性能。由于Cl-BT单元的强吸电子能力和EDOT单元的强给电子特性,所得Cl-EDOT前驱体可以在较低电位(0.71 V Ag/AgCl)下电聚合成高质量杂化聚合物薄膜,并具有良好的性能。氧化还原活性和更好的氧化还原稳定性。光谱电化学表明,所形成的低带隙 P(Cl-EDOT) 表现出从淡蓝色到灰色的可逆变色性质,并具有适度的光学对比度 (13.9%)、快速的响应时间 (0.6 s) 和良好的着色效率 ( 126.4 cm C) 在近红外区域,这是从动力学研究中测得的。通过优化测试电解质和溶液,其电致变色性能进一步提高(光学对比度:25.6%,响应时间:0.25 s,显色效率:275.9 C·cm)。 P(Cl-EDOT)良好的氧化还原稳定性和电致变色性能使其成为构建电致变色器件的良好候选者,并且Cl-BT单元应该成为设计高性能近红外电致变色材料的有前途的构建模块。
更新日期:2024-03-22
中文翻译:

不同给体对DA型氯化聚合物电化学行为和电致变色性能的影响
氯化已被证明是调节有机半导体材料能级和光电性能的有效方法,与氟化类似物相比,其合成成本更低,原材料成本也更低。本工作选择5-氯苯并[c][1,2,5]噻二唑(Cl-BT)单元作为新的受体单元,设计了两种新型DA型电致变色共轭聚合物:P(Cl-T)和P(Cl-EDOT)。采用电化学聚合技术和光谱电化学研究了上述氯化聚合物的电化学行为和电致变色性能。由于Cl-BT单元的强吸电子能力和EDOT单元的强给电子特性,所得Cl-EDOT前驱体可以在较低电位(0.71 V Ag/AgCl)下电聚合成高质量杂化聚合物薄膜,并具有良好的性能。氧化还原活性和更好的氧化还原稳定性。光谱电化学表明,所形成的低带隙 P(Cl-EDOT) 表现出从淡蓝色到灰色的可逆变色性质,并具有适度的光学对比度 (13.9%)、快速的响应时间 (0.6 s) 和良好的着色效率 ( 126.4 cm C) 在近红外区域,这是从动力学研究中测得的。通过优化测试电解质和溶液,其电致变色性能进一步提高(光学对比度:25.6%,响应时间:0.25 s,显色效率:275.9 C·cm)。 P(Cl-EDOT)良好的氧化还原稳定性和电致变色性能使其成为构建电致变色器件的良好候选者,并且Cl-BT单元应该成为设计高性能近红外电致变色材料的有前途的构建模块。



























 京公网安备 11010802027423号
京公网安备 11010802027423号