当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing Local pH Change during Electrode Oxidation of TEMPO Derivative: Implication of Redox-Induced Acidity Alternation by Imidazolium-Linker Functional Groups
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.analchem.3c05796 Jeongmin Yeo , Kyungmi Kim 1, 2 , Seung Jae Kwak 3 , Mi Song Kim 1 , Jung Hoon Yang 4 , Won Bo Lee 3 , YongJoo Kim 5, 6 , Junghyun Chae 1 , Jinho Chang
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-28 , DOI: 10.1021/acs.analchem.3c05796 Jeongmin Yeo , Kyungmi Kim 1, 2 , Seung Jae Kwak 3 , Mi Song Kim 1 , Jung Hoon Yang 4 , Won Bo Lee 3 , YongJoo Kim 5, 6 , Junghyun Chae 1 , Jinho Chang
Affiliation
|
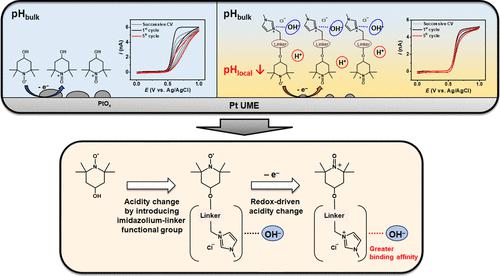
The chemical degradation of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-based aqueous energy storage and catalytic systems is pH sensitive. Herein, we voltammetrically monitor the local pH (pHlocal) at a Pt ultramicroelectrode (UME) upon electro-oxidation of imidazolium-linker functionalized TEMPO and show that its decrease is associated with the greater acidity of the cationic (oxidized) rather than radical (reduced) form of TEMPO. The protons that drive the decrease in pH arise from hydrolysis of the conjugated imidazolium-linker functional group of 4-[2-(N-methylimidazolium)acetoxy]-2,2,6,6-tetramethylpiperidine-1-oxyl chloride (MIMAcO-T), which was studied in comparison with 4-hydroxyl-TEMPO (4-OH-T). Voltammetric hysteresis is observed during the electrode oxidation of 4-OH-T and MIMAcO-T at a Pt UME in an unbuffered aqueous solution. The hysteresis arises from the pH-dependent formation and dissolution of Pt oxides, which interact with pHlocal in the vicinity of the UME. We find that electrogenerated MIMAcO-T+ significantly influences pHlocal, whereas 4-OH-T+ does not. Finite element analysis reveals that the thermodynamic and kinetic acid–base properties of MIMAcO-T+ are much more favorable than those of its reduced counterpart. Imidazolium-linker functionalized TEMPO molecules comprising different linking groups were also investigated. Reduced TEMPO molecules with carbonyl linkers behave as weak acids, whereas those with alkyl ether linkers do not. However, oxidized TEMPO+ molecules with alkyl ether linkers exhibit more facile acid–base kinetics than those with carbonyl ones. Density functional theory calculations confirm that OH– adduct formation on the imidazolium-linker functional group of TEMPO is responsible for the difference in the acid–base properties of the reduced and oxidized forms.
中文翻译:

探测 TEMPO 衍生物电极氧化过程中的局部 pH 变化:咪唑鎓连接基团官能团氧化还原诱导的酸度变化的影响
基于 2,2,6,6-四甲基哌啶-1-氧基 (TEMPO) 的水性储能和催化系统的化学降解对 pH 值敏感。在此,我们通过伏安法监测了咪唑鎓连接体官能化 TEMPO 电氧化时 Pt 超微电极 (UME) 的局部 pH (pH local ),并表明其降低与阳离子(氧化)而不是自由基的较高酸度相关(简化)形式的 TEMPO。驱动 pH 值降低的质子来自于 4-[2-( N-甲基咪唑鎓)乙酰氧基]-2,2,6,6-四甲基哌啶-1-氧氯 (MIMAcO-)的共轭咪唑鎓连接基团的水解。 T),与 4-羟基-TEMPO (4-OH-T) 进行比较研究。在未缓冲的水溶液中,Pt UME 上 4-OH-T 和 MIMAcO-T 的电极氧化过程中观察到伏安滞后现象。滞后现象是由 Pt 氧化物的 pH 依赖性形成和溶解引起的,Pt 氧化物与UME 附近的局部pH 值相互作用。我们发现电生成的 MIMAcO-T +显着影响局部pH 值,而 4-OH-T + 则不然。有限元分析表明,MIMAcO-T +的热力学和动力学酸碱性质比其还原对应物优越得多。还研究了包含不同连接基团的咪唑鎓连接基官能化的 TEMPO 分子。具有羰基连接基的还原 TEMPO 分子表现为弱酸,而具有烷基醚连接基的还原分子则不然。然而,具有烷基醚连接基的氧化 TEMPO + 分子比具有羰基连接基的氧化 TEMPO +分子表现出更容易的酸碱动力学。密度泛函理论计算证实,TEMPO 的咪唑鎓连接基团上形成的 OH加合物是还原形式和氧化形式的酸碱性质差异的原因。
更新日期:2024-03-28
中文翻译:

探测 TEMPO 衍生物电极氧化过程中的局部 pH 变化:咪唑鎓连接基团官能团氧化还原诱导的酸度变化的影响
基于 2,2,6,6-四甲基哌啶-1-氧基 (TEMPO) 的水性储能和催化系统的化学降解对 pH 值敏感。在此,我们通过伏安法监测了咪唑鎓连接体官能化 TEMPO 电氧化时 Pt 超微电极 (UME) 的局部 pH (pH local ),并表明其降低与阳离子(氧化)而不是自由基的较高酸度相关(简化)形式的 TEMPO。驱动 pH 值降低的质子来自于 4-[2-( N-甲基咪唑鎓)乙酰氧基]-2,2,6,6-四甲基哌啶-1-氧氯 (MIMAcO-)的共轭咪唑鎓连接基团的水解。 T),与 4-羟基-TEMPO (4-OH-T) 进行比较研究。在未缓冲的水溶液中,Pt UME 上 4-OH-T 和 MIMAcO-T 的电极氧化过程中观察到伏安滞后现象。滞后现象是由 Pt 氧化物的 pH 依赖性形成和溶解引起的,Pt 氧化物与UME 附近的局部pH 值相互作用。我们发现电生成的 MIMAcO-T +显着影响局部pH 值,而 4-OH-T + 则不然。有限元分析表明,MIMAcO-T +的热力学和动力学酸碱性质比其还原对应物优越得多。还研究了包含不同连接基团的咪唑鎓连接基官能化的 TEMPO 分子。具有羰基连接基的还原 TEMPO 分子表现为弱酸,而具有烷基醚连接基的还原分子则不然。然而,具有烷基醚连接基的氧化 TEMPO + 分子比具有羰基连接基的氧化 TEMPO +分子表现出更容易的酸碱动力学。密度泛函理论计算证实,TEMPO 的咪唑鎓连接基团上形成的 OH加合物是还原形式和氧化形式的酸碱性质差异的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号