Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-28 , DOI: 10.3762/bjoc.20.61 Dāgs Dāvis Līpiņš , Andris Jeminejs , Una Ušacka , Anatoly Mishnev , Māris Turks , Irina Novosjolova
Abstract
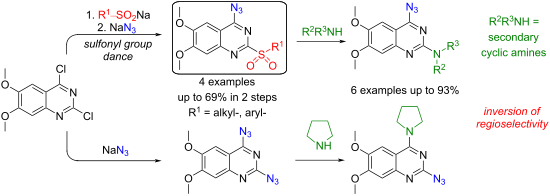
2-Chloro-4-sulfonylquinazolines undergo functional group swap when treated with an azide nucleophile: 1) the azide replaces the sulfonyl group at the C4 position; 2) the intrinsic azide–tetrazole tautomeric equilibrium directs the nucleofugal sulfinate from the first step to replace chloride at the C2 position. This transformation is effective with quinazolines bearing electron-rich substituents. Therefore, the title transformations are demonstrated on the 6,7-dimethoxyquinazoline core, which is present in pharmaceutically active substances. The methodology application is showcased by transforming the obtained 4-azido-6,7-dimethoxy-2-sulfonylquinazolines into the α1-adrenoceptor blockers terazosin and prazosin by further C2-selective SNAr reaction and azide reduction.
Beilstein J. Org. Chem. 2024, 20, 675–683. doi:10.3762/bjoc.20.61
中文翻译:

通过叠氮-四唑互变异构平衡进行区域选择性喹唑啉 C2 修饰
摘要
当用叠氮化物亲核试剂处理时,2-氯-4-磺酰基喹唑啉会发生官能团交换:1) 叠氮化物取代 C4 位的磺酰基; 2) 内在的叠氮化物-四唑互变异构平衡引导第一步中的离核亚磺酸盐取代 C2 位处的氯化物。这种转变对于带有富电子取代基的喹唑啉是有效的。因此,标题转换在药物活性物质中存在的 6,7-二甲氧基喹唑啉核心上得到证实。通过进一步的C2选择性SN Ar反应和叠氮化物还原,将获得的4-叠氮基-6,7-二甲氧基-2-磺酰基喹唑啉转化为α1-肾上腺素受体阻滞剂特拉唑嗪和哌唑嗪,展示了该方法的应用。

贝尔斯坦 J. 组织。化学。 2024, 20, 675–683。 doi:10.3762/bjoc.20.61



























 京公网安备 11010802027423号
京公网安备 11010802027423号