Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2024-03-27 , DOI: 10.1002/adsc.202400043 Sergio Otero Riesgo 1 , Jesús A. Varela 2 , Carlos Saá 2
|
Introduction
Pyrene, a polycyclic aromatic hydrocarbon (PAH) that contains four fused benzene rings, is well recognized by its unique optical properties, good stability and hole transporting ability that makes very attractive for applications in organic electronic devices (Figure 1).1 Their planar isoelectronic N-doped cyclopenta[c,d]phenalene derivatives, ullazines, have received extensive interest in the field of dye-sensitized and perovskite solar cells (DSSCs and PSCs)2 for their excellent performance as light harvesting moieties and electron donor abilities (Figure 1).3

Pyrene and Ullazines.
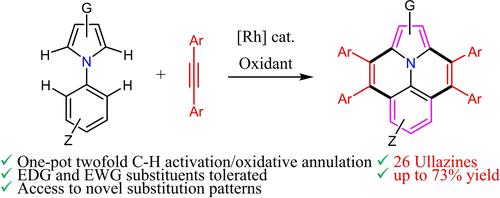
Most typical synthetic routes to ullazines2b, 4 often rely on the use of halogenated N-arylpyrroles2b, 4c-4e, 4j (Scheme 1a, eq 1) but, however, these methods present limitations in substrate scope and difficulties in achieving densely functionalized ullazines, underscoring the need for improved synthetic strategies. On the contrary, synthetic routes based on metal-catalyzed C−H activation/oxidative annulation of non-halogenated N-arylpyrroles have been scarcely developed. A pioneering stepwise chromium-mediated synthesis of ullazines from N-arylpyrroles and alkynes has been described by Takahashi (Scheme 1a, eq 2).4a Zhou recently reported the synthesis of a little number of ullazines by Rh(III)-catalyzed oxidative annulation of pyrroloquinolines with diphenylacetylene5 based on early described Dong/Chen's conditions for single C−H activation/oxidative annulation of N-arylpyrroles to pyrroloquinolines (Scheme 1a, eq 3).6 It is worth to mention that an excess of valuable N-arylpyrrole partners were required for these successful cycloadditions. To the best of our knowledge, one-pot synthesis of ullazines starting from N-arylpyrroles has not yet been developed.

Synthetic routes to ullazines.
Herein, we report a novel route to functionalized ullazines based on a one-pot Rh(III)-catalyzed twofold C−H activation/oxidative annulation of N-arylpyrroles with alkynes. The reaction is tolerant with arylalkynes and N-arylpyrroles bearing EDG and EWG groups including halogens (Cl, Br, F) in different positions of the ullazine skeleton available for synthetic manipulations at specific sites (Scheme 1b).
中文翻译:

一锅法 Rh(III) 催化 N-芳基吡咯与炔烃的双重 C−H 活化/氧化成荧光尿素
介绍
芘是一种含有四个稠合苯环的多环芳烃 (PAH),因其独特的光学性质、良好的稳定性和空穴传输能力而受到广泛认可,在有机电子器件中的应用非常有吸引力(图 1)。1他们的平面等电子氮掺杂环戊二烯[ c , d ]苯酚衍生物乌拉嗪在染料敏化和钙钛矿太阳能电池(DSSC 和 PSC)领域受到广泛关注2因其作为光捕获部分和电子供体的优异性能能力(图1)。3

芘和乌拉津。
最典型的尿拉嗪2b, 4合成路线通常依赖于使用卤代N-芳基吡咯2b, 4c-4e, 4j(方案 1a, eq 1),但是,这些方法存在底物范围的限制以及实现密集功能化的困难拉嗪,强调需要改进的合成策略。相反,基于非卤代N-芳基吡咯的金属催化CH活化/氧化成环的合成路线几乎尚未开发。Takahashi 描述了一种开创性的铬介导的从N-芳基吡咯和炔烃逐步合成尿拉嗪的方法(方案 1a,方程式 2)。 4a Zhou最近报道了基于Dong/Chen早期描述的N-芳基吡咯单CH活化/氧化环化为吡咯并喹啉的条件,通过Rh(III)催化吡咯喹啉与二苯乙炔5的氧化环化合成了少量尿拉嗪(方案 1a,等式 3)。6值得一提的是,这些成功的环加成反应需要过量的有价值的N -芳基吡咯伴侣。据我们所知,尚未开发出以N-芳基吡咯为原料的一锅法合成尿拉嗪的方法。

乌拉嗪的合成路线。
在此,我们报告了一种基于一锅法 Rh(III) 催化的N-芳基吡咯与炔烃的双重 C−H 活化/氧化成环的功能化尿拉嗪的新途径。该反应能够耐受带有 EDG 和 EWG 基团的芳基炔和N-芳基吡咯,包括位于尿拉嗪骨架不同位置的卤素(Cl、Br、F),可用于在特定位点进行合成操作(方案 1b)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号