当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ab initio thermodynamic and kinetic modeling of molecular adsorption and reaction properties on PuO2(111) surface under exposure to environmental gases
Surface Science ( IF 1.9 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.susc.2024.122482 Jinfan Chen , Jun Tang , Pengchuang Liu , Ruizhi Qiu
Surface Science ( IF 1.9 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.susc.2024.122482 Jinfan Chen , Jun Tang , Pengchuang Liu , Ruizhi Qiu
|
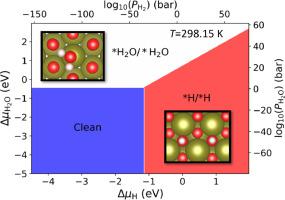
Adsorption and reaction properties of environmental gases including O, H, and HO on the PuO(111) surface were studied via density functional theory simulations along with thermodynamic and kinetic analysis. Simulation results show that the stoichiometric PuO(111) remains intact under O atmosphere and extremely low or high O pressure is required to form oxygen vacancy or adsorbed-O on the surface. The HO prefers to stay as molecular state when adsorbing on PuO(111) and a relatively high humidity is required for HO to be stably binding on the surface. For H interaction with PuO, the dissociative adsorption of H molecule induces reduction of Pu(IV) ions to Pu(III), and remains thermodynamically stable at H pressure as low as ∼10 bar under room temperature. Kinetic modeling shows that at temperature below 350 K, the PuO(111) surface is mainly covered by OH species when exposing to H environment while bare metal sites appear with increased temperature and reaction time.
中文翻译:

环境气体暴露下 PuO2(111) 表面分子吸附和反应特性的从头热力学和动力学建模
通过密度泛函理论模拟以及热力学和动力学分析,研究了O、H和H2O等环境气体在PuO(111)表面的吸附和反应特性。模拟结果表明,化学计量的PuO(111)在O气氛下保持完整,并且需要极低或极高的O压力才能在表面形成氧空位或吸附O。当吸附在 PuO(111) 上时,H2O 更倾向于保持分子状态,并且 H2O 需要相对较高的湿度才能稳定地结合在表面上。对于H与PuO的相互作用,H分子的解离吸附导致Pu(IV)离子还原为Pu(III),并且在室温下低至〜10 bar的H压力下保持热力学稳定。动力学模型表明,在低于350 K的温度下,当暴露于H环境中时,PuO(111)表面主要被OH物质覆盖,而随着温度和反应时间的增加,出现裸露的金属位点。
更新日期:2024-03-16
中文翻译:

环境气体暴露下 PuO2(111) 表面分子吸附和反应特性的从头热力学和动力学建模
通过密度泛函理论模拟以及热力学和动力学分析,研究了O、H和H2O等环境气体在PuO(111)表面的吸附和反应特性。模拟结果表明,化学计量的PuO(111)在O气氛下保持完整,并且需要极低或极高的O压力才能在表面形成氧空位或吸附O。当吸附在 PuO(111) 上时,H2O 更倾向于保持分子状态,并且 H2O 需要相对较高的湿度才能稳定地结合在表面上。对于H与PuO的相互作用,H分子的解离吸附导致Pu(IV)离子还原为Pu(III),并且在室温下低至〜10 bar的H压力下保持热力学稳定。动力学模型表明,在低于350 K的温度下,当暴露于H环境中时,PuO(111)表面主要被OH物质覆盖,而随着温度和反应时间的增加,出现裸露的金属位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号