当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The reduction mechanism of C1 product from carbon dioxide catalyzed by Ni-doped g-C3N4
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.mcat.2024.114064 Shuwei Zhang , Huining Feng , Chenyu Li , Xindi Cao , Hui Li , Yang Wu
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.mcat.2024.114064 Shuwei Zhang , Huining Feng , Chenyu Li , Xindi Cao , Hui Li , Yang Wu
|
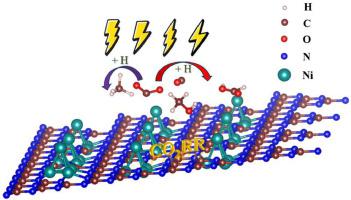
This work employs density functional theory (DFT) to scrutinize the catalytic efficacy of nano nickel (Ni) clusters supported by graphitic carbon nitride (Ni@g-CN, where n ranges from 1 to 6) in the context of the CO reduction reaction (CORR). Structural examination revealed that Ni@g-CN possesses a substantial binding energy (-1.63 eV to -7.72 eV), confirming the structural stability of the catalyst in the CORR. Electronic structure analysis revealed a pronounced orbital overlap near the Fermi level between the 3d orbital of Ni atoms and the 2p orbital of adjacent cavity nitrogen atoms in Ni@g-CN. Further insights are gleaned from the calculations of the Bader charge and energy band, indicating significant charge transfer and band gap alteration, suggesting enhanced conductivity due to Ni doping on g-CN. The catalytic performance in the CORR is predominantly influenced by the size of the doped Ni clusters. The Ni@g-CN cluster demonstrated optimal efficiency in producing formic acid (HCOOH) with a limiting potential of -0.12 V. In contrast, the Ni@g-CN cluster excels in methane (CH) formation, with a limiting potential of -0.35 V. Additionally, these catalysts exhibit marked inhibition of the hydrogen evolution reaction, further underscoring their potential in CORR applications.
中文翻译:

Ni掺杂g-C3N4催化二氧化碳还原C1产物的机理
这项工作采用密度泛函理论 (DFT) 来研究石墨氮化碳 (Ni@g-CN,其中 n 范围为 1 至 6) 支撑的纳米镍 (Ni) 簇在 CO 还原反应中的催化功效 (校正)。结构检查表明Ni@g-CN具有相当大的结合能(-1.63 eV至-7.72 eV),证实了CO2RR中催化剂的结构稳定性。电子结构分析表明,Ni@g-CN 中 Ni 原子的 3d 轨道与相邻腔氮原子的 2p 轨道在费米能级附近存在明显的轨道重叠。从 Bader 电荷和能带的计算中获得了进一步的见解,表明电荷转移和带隙变化显着,表明 g-CN 上的 Ni 掺杂导致电导率增强。 CORR 中的催化性能主要受掺杂 Ni 团簇尺寸的影响。 Ni@g-CN簇在生产甲酸(HCOOH)方面表现出最佳效率,极限电势为-0.12 V。相比之下,Ni@g-CN簇在甲烷(CH)形成方面表现出色,极限电势为- 0.35 V。此外,这些催化剂对析氢反应具有显着的抑制作用,进一步凸显了它们在 CORR 应用中的潜力。
更新日期:2024-03-29
中文翻译:

Ni掺杂g-C3N4催化二氧化碳还原C1产物的机理
这项工作采用密度泛函理论 (DFT) 来研究石墨氮化碳 (Ni@g-CN,其中 n 范围为 1 至 6) 支撑的纳米镍 (Ni) 簇在 CO 还原反应中的催化功效 (校正)。结构检查表明Ni@g-CN具有相当大的结合能(-1.63 eV至-7.72 eV),证实了CO2RR中催化剂的结构稳定性。电子结构分析表明,Ni@g-CN 中 Ni 原子的 3d 轨道与相邻腔氮原子的 2p 轨道在费米能级附近存在明显的轨道重叠。从 Bader 电荷和能带的计算中获得了进一步的见解,表明电荷转移和带隙变化显着,表明 g-CN 上的 Ni 掺杂导致电导率增强。 CORR 中的催化性能主要受掺杂 Ni 团簇尺寸的影响。 Ni@g-CN簇在生产甲酸(HCOOH)方面表现出最佳效率,极限电势为-0.12 V。相比之下,Ni@g-CN簇在甲烷(CH)形成方面表现出色,极限电势为- 0.35 V。此外,这些催化剂对析氢反应具有显着的抑制作用,进一步凸显了它们在 CORR 应用中的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号