当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic possibility analysis of CH3COOH synthesis from CH4 and CO2
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.mcat.2024.114090 Xin-Yu Zhang , Shuai Bian , Hai-Feng Tian , Zhi-Feng Yan , Jia-Yao Feng , Lei Liu , Wei Huang , Zhi-Jun Zuo
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.mcat.2024.114090 Xin-Yu Zhang , Shuai Bian , Hai-Feng Tian , Zhi-Feng Yan , Jia-Yao Feng , Lei Liu , Wei Huang , Zhi-Jun Zuo
|
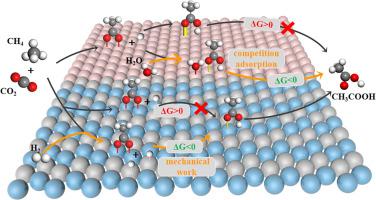
CHCOOH synthesis from CH and CO is a green and 100% atom-efficient reaction, but it is a thermodynamically unfavorable reaction. To better understand the thermodynamic limits and implementation methods of CHCOOH synthesis, thermodynamics is systematically studied by using HSC chemistry and density functional theory. It is found that the reaction is thermodynamically unfavorable in the homogeneous phase at 298 ∼1000 K and 0.1∼30 MPa, and the maximum equilibrium amount of CHCOOH is 6.0 × 10%. The bond strength between CHCOOH and the active site is the key factor affecting the thermodynamics of this reaction in the heterogeneous phase. If the bond strength between CHCOOH and the active site is weak, H can break the thermodynamic bottleneck, of which the mechanical work of the pressure (H (supplied)) overcomes the thermodynamic barrier. On the contrary, HO can break the thermodynamic bottleneck due to the competition adsorption between HO and CHCOOH.
中文翻译:

CH4和CO2合成CH3COOH的热力学可能性分析
由CH和CO合成CH3COOH是一种绿色且100%原子效率的反应,但它是一种热力学不利的反应。为了更好地理解CHCOOH合成的热力学极限和实现方法,利用HSC化学和密度泛函理论对热力学进行了系统的研究。结果发现,在298~1000 K、0.1~30 MPa条件下,该反应在均相中热力学不利,CHCOOH的最大平衡量为6.0×10%。 CHCOOH与活性位点之间的键合强度是影响多相反应热力学的关键因素。如果CHCOOH与活性位点之间的键合强度较弱,H可以打破热力学瓶颈,其中压力(H(提供))的机械功克服了热力学势垒。相反,由于H2O和CHCOOH之间的竞争吸附,H2O可以打破热力学瓶颈。
更新日期:2024-03-29
中文翻译:

CH4和CO2合成CH3COOH的热力学可能性分析
由CH和CO合成CH3COOH是一种绿色且100%原子效率的反应,但它是一种热力学不利的反应。为了更好地理解CHCOOH合成的热力学极限和实现方法,利用HSC化学和密度泛函理论对热力学进行了系统的研究。结果发现,在298~1000 K、0.1~30 MPa条件下,该反应在均相中热力学不利,CHCOOH的最大平衡量为6.0×10%。 CHCOOH与活性位点之间的键合强度是影响多相反应热力学的关键因素。如果CHCOOH与活性位点之间的键合强度较弱,H可以打破热力学瓶颈,其中压力(H(提供))的机械功克服了热力学势垒。相反,由于H2O和CHCOOH之间的竞争吸附,H2O可以打破热力学瓶颈。



























 京公网安备 11010802027423号
京公网安备 11010802027423号