当前位置:
X-MOL 学术
›
Mol. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monoethanolamine assisted CO2 hydrogenation to methanol – A computational study
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.mcat.2024.114091 Rachid Hadjadj , Imre G. Csizmadia , Hadeer Q. Waleed , Dalal K. Thbayh , Béla Viskolcz , Béla Fiser
Molecular Catalysis ( IF 4.6 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.mcat.2024.114091 Rachid Hadjadj , Imre G. Csizmadia , Hadeer Q. Waleed , Dalal K. Thbayh , Béla Viskolcz , Béla Fiser
|
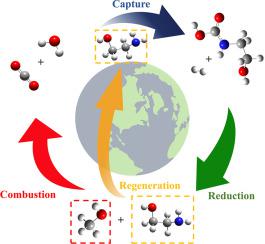
Carbon dioxide emission to the atmosphere has to be reduced which can be done by utilizing CO in the synthesis of added value products. At the same time this will lead to a process which can help to store renewable energy. The use of hydrogen produced from the electrolysis of water in carbon dioxide reduction to methanol could be an efficient way to store energy and to convert CO into an added value product. The synthesis of methanol from CO is usually performed catalytically in gas phase. Many scientists nowadays expressed interest in testing the feasibility of the reaction also in aqueous phase. In this direction, monoethanolamine (MEA) can be used as a solvent for capturing and trapping carbon dioxide in an aqueous phase. In this work, the hydrogenation of (2-hydroxyethyl) carbamic acid (HO-(CH)-NH-COOH) which is one of the produced species during the capture process has been investigated by using computational tools. The uncatalyzed and catalyzed-like hydrogenation mechanisms leading to methanol (and MEA+water) as a product has been studied by using high level calculations in aqueous phase. The mechanisms have been described at the molecular level to provide a deeper understanding of the processes. The calculations indicate that the highest barrier height in the catalyzed-like process is only 114.67 kJ/mol, which is 227.71 kJ/mol lower than the corresponding step in the uncatalyzed mechanism. Furthermore, the energy storage efficiency of the catalyzed-like process is 96.68 %, which is 7.5 times more efficient than the uncatalyzed mechanism.
中文翻译:

单乙醇胺辅助二氧化碳加氢生成甲醇——一项计算研究
必须减少向大气中排放的二氧化碳,这可以通过在增值产品的合成中利用二氧化碳来实现。同时,这将导致一个有助于储存可再生能源的过程。使用电解水产生的氢气将二氧化碳还原为甲醇可能是储存能量并将二氧化碳转化为附加值产品的有效方法。由CO合成甲醇通常在气相催化下进行。如今许多科学家表示有兴趣在水相中测试该反应的可行性。在这个方向上,单乙醇胺(MEA)可以用作在水相中捕获和捕集二氧化碳的溶剂。在这项工作中,使用计算工具研究了捕获过程中产生的物质之一(2-羟乙基)氨基甲酸(HO-(CH)-NH-COOH)的氢化。通过在水相中使用高级计算,研究了产生甲醇(和 MEA+水)产品的非催化和类催化氢化机制。这些机制已在分子水平上进行了描述,以便更深入地了解该过程。计算表明,类催化过程中的最高势垒高度仅为114.67 kJ/mol,比非催化机制中的相应步骤低227.71 kJ/mol。此外,类催化过程的储能效率为96.68%,比非催化机制高7.5倍。
更新日期:2024-03-29
中文翻译:

单乙醇胺辅助二氧化碳加氢生成甲醇——一项计算研究
必须减少向大气中排放的二氧化碳,这可以通过在增值产品的合成中利用二氧化碳来实现。同时,这将导致一个有助于储存可再生能源的过程。使用电解水产生的氢气将二氧化碳还原为甲醇可能是储存能量并将二氧化碳转化为附加值产品的有效方法。由CO合成甲醇通常在气相催化下进行。如今许多科学家表示有兴趣在水相中测试该反应的可行性。在这个方向上,单乙醇胺(MEA)可以用作在水相中捕获和捕集二氧化碳的溶剂。在这项工作中,使用计算工具研究了捕获过程中产生的物质之一(2-羟乙基)氨基甲酸(HO-(CH)-NH-COOH)的氢化。通过在水相中使用高级计算,研究了产生甲醇(和 MEA+水)产品的非催化和类催化氢化机制。这些机制已在分子水平上进行了描述,以便更深入地了解该过程。计算表明,类催化过程中的最高势垒高度仅为114.67 kJ/mol,比非催化机制中的相应步骤低227.71 kJ/mol。此外,类催化过程的储能效率为96.68%,比非催化机制高7.5倍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号