当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spatiotemporal tracking of intracellular nanoparticles using complementary imaging systems reveals acute ferroptosis triggered by burst reduction of ferric ions
Nano Today ( IF 17.4 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.nantod.2024.102242 Chan-Gi Pack , Min Kyo Jung , Kyunghwan Kim , Woojung Yoo , Minjong Kim , Minju Cho , Myoung-Hee Kang , Sanghwa Lee , Jisu Im , In Ki Kim , Sang-Wook Lee , Jun Ki Kim , Jinmyoung Joo
Nano Today ( IF 17.4 ) Pub Date : 2024-03-29 , DOI: 10.1016/j.nantod.2024.102242 Chan-Gi Pack , Min Kyo Jung , Kyunghwan Kim , Woojung Yoo , Minjong Kim , Minju Cho , Myoung-Hee Kang , Sanghwa Lee , Jisu Im , In Ki Kim , Sang-Wook Lee , Jun Ki Kim , Jinmyoung Joo
|
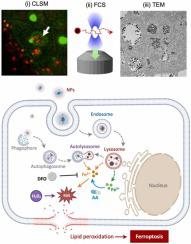
Uptake and intracellular trafficking of nanoparticles are tightly regulated by their interactions with cellular organelles and physiological microenvironment. Although the dynamic physicochemical reactions at the interface of nanoparticles and cells ultimately determine the intracellular distribution and fate, microscopic tracing and quantitative analysis of the nanoparticles have been hampered by the limited resolution associated with individual nanoparticle trafficking. Herein, we report spatiotemporal investigations on autophagic clearance of biodegradable iron oxide-silica core-shell nanoparticles in terms of intracellular trafficking and ionic dissolution at a single cell level using multimodal imaging systems. By combining transmission electron microscopy and super-resolution confocal laser scanning microscopy with fluorescence correlation spectroscopy, the complementary imaging analysis exclusively shows the intracellular uptake, endosomal fusion and biodegradative clearance, leading to identify the step-by-step endocytic transport pathway and autophagic degradation pathways. Tracing the intracellular trafficking of nanoparticles reveals that they are spontaneously transported from endosomes to lysosomes, and transiently stimulate autophagy while maintaining cell viability. While protecting iron oxide core, the silica shell is gradually degraded during endocytosis and autophagic clearance, resulting in ionic dissolution of iron oxide in acidic environment. Moreover, burst reduction of ferric ions by adding ascorbic acid readily triggers acute ferroptosis owing to rapid supplement of ferrous ions and Fenton reaction in cancer cells. The complementary imaging strategy provides insights into the design of biocompatible nanomedicines for cellular delivery and the degradative mechanisms beyond the intracellular fate.
中文翻译:

使用互补成像系统对细胞内纳米颗粒进行时空追踪揭示了由三价铁离子爆发还原引发的急性铁死亡
纳米颗粒的摄取和细胞内运输受到它们与细胞器和生理微环境的相互作用的严格调节。尽管纳米颗粒和细胞界面处的动态物理化学反应最终决定了细胞内的分布和命运,但纳米颗粒的微观追踪和定量分析受到与单个纳米颗粒运输相关的有限分辨率的阻碍。在此,我们报告了使用多模态成像系统在单细胞水平上对可生物降解的氧化铁-二氧化硅核-壳纳米颗粒的自噬清除在单细胞水平上的细胞内运输和离子溶解进行的时空研究。通过将透射电子显微镜、超分辨率共焦激光扫描显微镜与荧光相关光谱相结合,互补成像分析独家显示细胞内摄取、内体融合和生物降解清除,从而确定逐步的内吞转运途径和自噬降解途径。追踪纳米粒子的细胞内运输表明,它们自发地从内体转运到溶酶体,并短暂刺激自噬,同时保持细胞活力。在保护氧化铁核的同时,二氧化硅壳在内吞作用和自噬清除过程中逐渐降解,导致氧化铁在酸性环境中离子溶解。此外,由于亚铁离子的快速补充和癌细胞中的芬顿反应,通过添加抗坏血酸来突然减少铁离子很容易引发急性铁死亡。互补成像策略为细胞递送的生物相容性纳米药物的设计以及细胞内命运之外的降解机制提供了见解。
更新日期:2024-03-29
中文翻译:

使用互补成像系统对细胞内纳米颗粒进行时空追踪揭示了由三价铁离子爆发还原引发的急性铁死亡
纳米颗粒的摄取和细胞内运输受到它们与细胞器和生理微环境的相互作用的严格调节。尽管纳米颗粒和细胞界面处的动态物理化学反应最终决定了细胞内的分布和命运,但纳米颗粒的微观追踪和定量分析受到与单个纳米颗粒运输相关的有限分辨率的阻碍。在此,我们报告了使用多模态成像系统在单细胞水平上对可生物降解的氧化铁-二氧化硅核-壳纳米颗粒的自噬清除在单细胞水平上的细胞内运输和离子溶解进行的时空研究。通过将透射电子显微镜、超分辨率共焦激光扫描显微镜与荧光相关光谱相结合,互补成像分析独家显示细胞内摄取、内体融合和生物降解清除,从而确定逐步的内吞转运途径和自噬降解途径。追踪纳米粒子的细胞内运输表明,它们自发地从内体转运到溶酶体,并短暂刺激自噬,同时保持细胞活力。在保护氧化铁核的同时,二氧化硅壳在内吞作用和自噬清除过程中逐渐降解,导致氧化铁在酸性环境中离子溶解。此外,由于亚铁离子的快速补充和癌细胞中的芬顿反应,通过添加抗坏血酸来突然减少铁离子很容易引发急性铁死亡。互补成像策略为细胞递送的生物相容性纳米药物的设计以及细胞内命运之外的降解机制提供了见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号